2 1 Monitoring Water Quality A Water Quality














- Slides: 16

2. 1 Monitoring Water Quality

A. Water Quality ¬water that looks clean may not be clean ¬there are many toxic dissolved substances ¬monitoring water quality is an accurate way of keeping track of what is in water systems ¬there are two indicators used to monitor water quality: biological and chemical slide 1 of 14

B. Biological Indicators ¬biological indicators alert scientists to potential environmental problems ¬the types of organisms that live in the water are an indication of conditions like the amount of dissolved oxygen and p. H slide 2 of 14

¬two main types of biological indicators are: 1. microorganisms eg) bacteria 2. aquatic invertebrates (animals without backbones) eg) shrimp, leeches, worms ¬high amounts of harmful bacteria like E. coli would result in water treatment being necessary slide 3 of 14

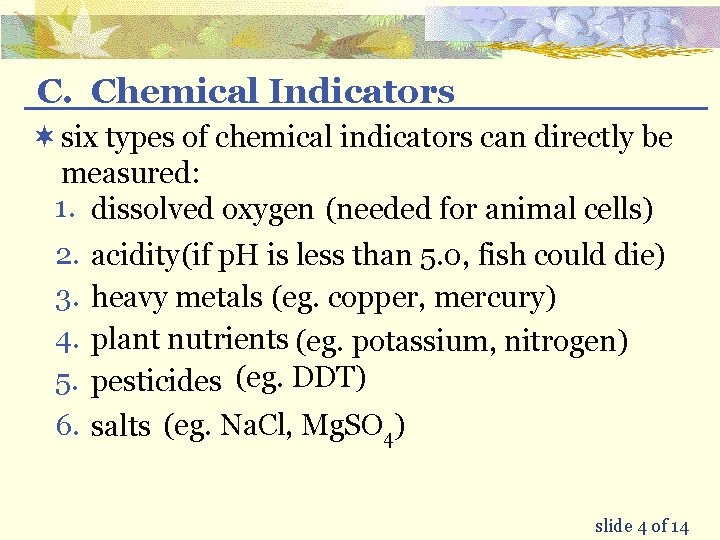
C. Chemical Indicators ¬ six types of chemical indicators can directly be measured: 1. dissolved oxygen (needed for animal cells) 2. acidity (if p. H is less than 5. 0, fish could die) 3. heavy metals (eg. copper, mercury) 4. plant nutrients (eg. potassium, nitrogen) 5. pesticides (eg. DDT) 6. salts (eg. Na. Cl, Mg. SO 4) slide 4 of 14

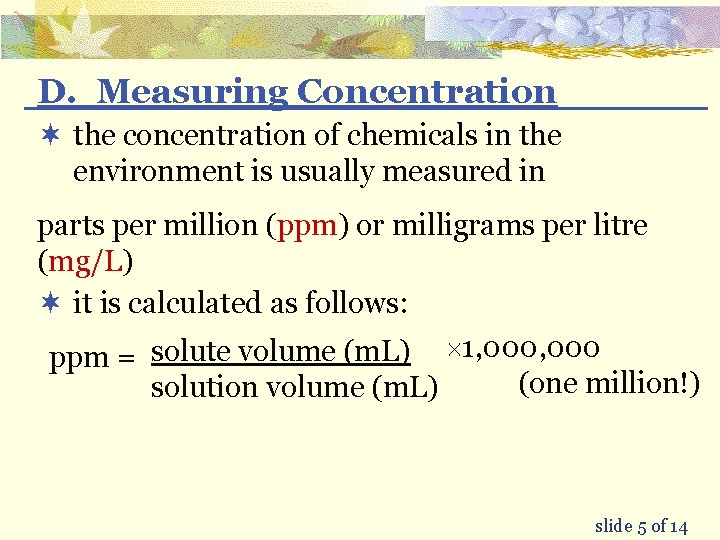
D. Measuring Concentration ¬ the concentration of chemicals in the environment is usually measured in parts per million (ppm) or milligrams per litre (mg/L) ¬ it is calculated as follows: ppm = solute volume (m. L) ´ 1, 000 (one million!) solution volume (m. L) slide 5 of 14

Example Calculate the concentration if 1 m. L chlorine is dissolved to make 10, 000 m. L of solution. ppm = solute volume (m. L) ´ 1, 000 solution volume (m. L) 1 m. L 10, 000 m. L = 100 ppm = ´ 1, 000 slide 6 of 14

E. Dissolved Oxygen ¬ oxygen dissolves in water by diffusion from air ¬dissolved oxygen is removed by: 1. decomposition (rotting) of organic material 2. cellular respiration (fish gills) slide 7 of 14

¬ level of dissolved O 2(g) depends on 4 factors: 1. temperature 2. wind/water turbulence 3. amount of photosynthesis 4. number of organisms slide 8 of 14

F. Nitrogen and Phosphorus ¬ plant growth is stimulated by nutrients such as nitrates and phosphates ¬ increasing the concentration of these nutrients in water increases plant production slide 9 of 14

¬ plants eventually die and decomposers (bacteria) break down the dead plants and use up a lot of dissolved O 2(g) ¬ the invertebrate and fish populations start to die off and you are left with leeches, worms and mosquitoes slide 10 of 14

G. Acidity ¬ normal rain has a p. H of 5. 6 while acid rain can be much lower (3. 0) ¬ as p. H goes down (more acidic) so does the amount of biodiversity ¬ most fish die if p. H goes below 4. 5 slide 11 of 14

¬acid can build up in snow and ice in the winter and then be released all at once in the spring…called spring acid shock ¬ this can weaken the eggs of organisms and kill their young slide 12 of 14

H. Heavy Metals ¬ heavy metals are metals with a minimum density of 5 g/cm 3 eg) lead, copper, mercury ¬ occur naturally in small amounts ¬ human activities like industries and farming tend to concentrate them in ecosystems ¬ they harm the normal development of humans and other organisms slide 13 of 14

I. Measuring Toxicity ¬ scientists use a measurement called LD 50 to measure toxicity ¬ LD 50 is the amount of a substance given all at once that causes 50% of a test group of animals to die ¬ the more toxic the substance, the lower the LD 50 number slide 14 of 14

n Assignment worksheet 5 and 6
 National water quality monitoring conference
National water quality monitoring conference National water quality monitoring council
National water quality monitoring council National water quality monitoring conference
National water quality monitoring conference National water quality monitoring conference
National water quality monitoring conference Water and water and water water
Water and water and water water Quality monitoring scorecard
Quality monitoring scorecard Quality monitoring scorecard
Quality monitoring scorecard Data quality monitoring framework
Data quality monitoring framework Wdqms
Wdqms Real time data quality monitoring
Real time data quality monitoring Permanent power quality monitoring equipment
Permanent power quality monitoring equipment Reservoir monitoring system
Reservoir monitoring system Reservoir water monitoring systems
Reservoir water monitoring systems Perform quality assurance
Perform quality assurance Plan quality management pmp
Plan quality management pmp Pmbok quality assurance vs quality control
Pmbok quality assurance vs quality control Process of nursing audit
Process of nursing audit