2 1 Ionic Bonding 1 of of 68
2. 1 Ionic Bonding 1 of of 68 20 © Boardworks Ltd 2005 2004

BOND JAMES Taken not shared

Learning Goals By the end of this lesson you will be able to: 1) Define what an ionic bond is 2) Be able to react a metal and a non-metal to create an ionic bond 3) Describe properties of ionic compounds
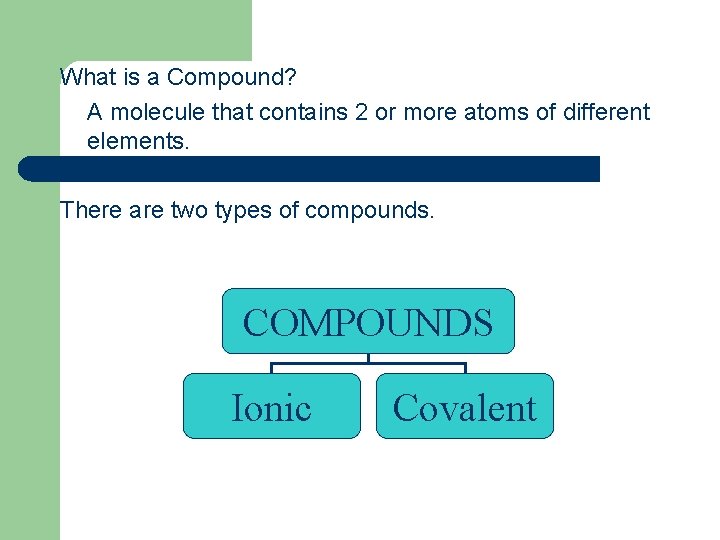
What is a Compound? A molecule that contains 2 or more atoms of different elements. There are two types of compounds. COMPOUNDS Ionic Covalent
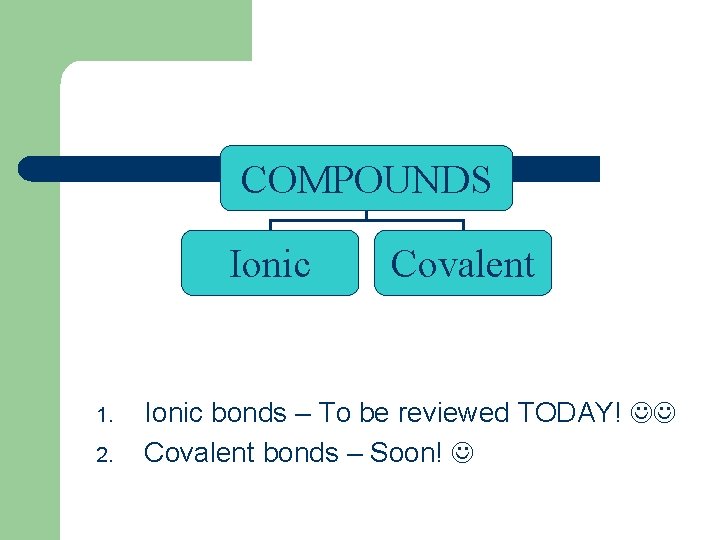
COMPOUNDS Ionic 1. 2. Covalent Ionic bonds – To be reviewed TODAY! Covalent bonds – Soon!

Full electron shells Atoms of noble gases, group 8, have completely full outer shells. This makes them very unreactive or stable. 2 nd shell holds a maximum of 1 st shell holds 8 electrons a maximum of 2 electrons 3 rd shell holds a maximum of 8 electrons 1 of 68 20 6 of © Boardworks Ltd 2005 2004

Atoms and electron changes Every atom would like to have a full outer shell like the noble gases. Atoms can get full outer electron shells by either gaining or losing electrons 1 of 68 20 7 of © Boardworks Ltd 2005 2004
From atoms to ions How can reactive metal atoms become stable positive ions? 1 of 68 20 8 of © Boardworks Ltd 2005 2004

Ionic Bonds: One Big Greedy Thief Dog! 1 of 68 20 9 of © Boardworks Ltd 2005 2004

Bonding: example 1 • Making sodium chloride – table salt!!! 1 10 of of 20 68 © Boardworks Ltd 2005 2004
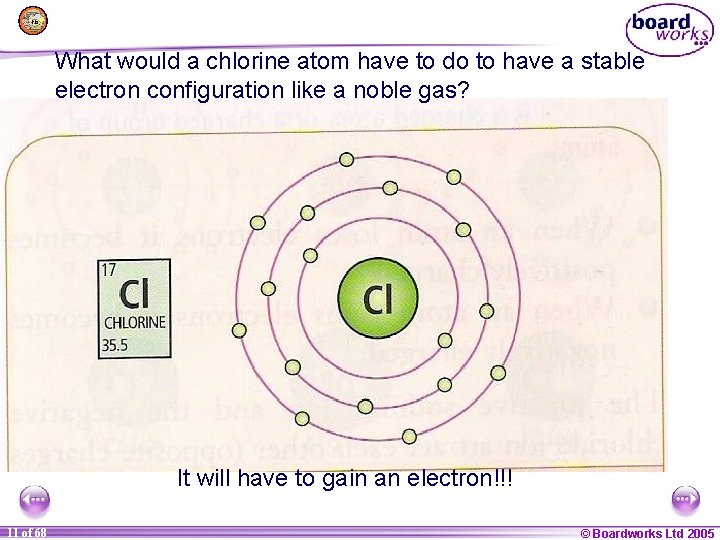
What would a chlorine atom have to do to have a stable electron configuration like a noble gas? It will have to gain an electron!!! 1 11 of of 20 68 © Boardworks Ltd 2005 2004
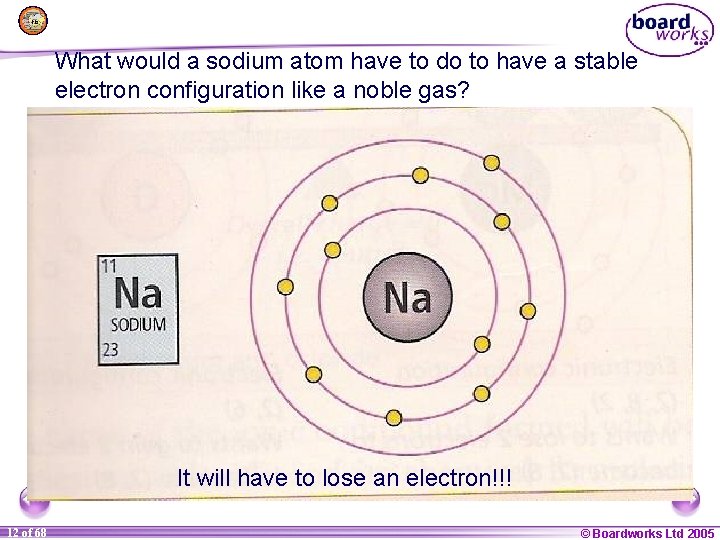
What would a sodium atom have to do to have a stable electron configuration like a noble gas? It will have to lose an electron!!! 1 12 of of 20 68 © Boardworks Ltd 2005 2004
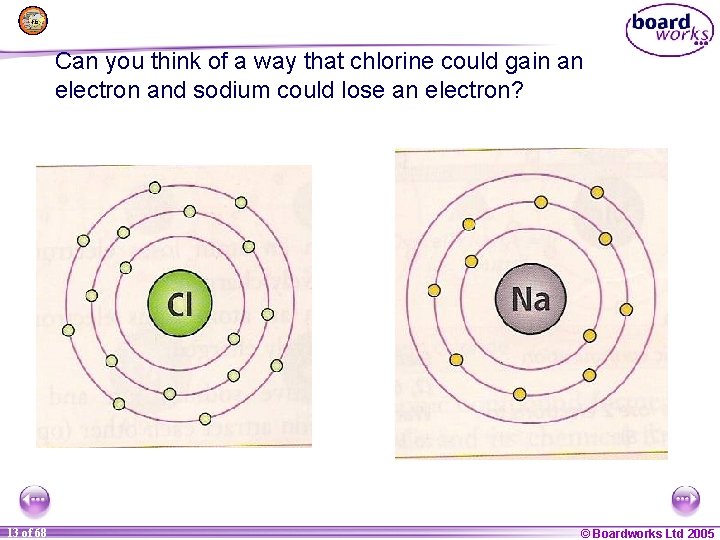
Can you think of a way that chlorine could gain an electron and sodium could lose an electron? 1 13 of of 20 68 © Boardworks Ltd 2005 2004
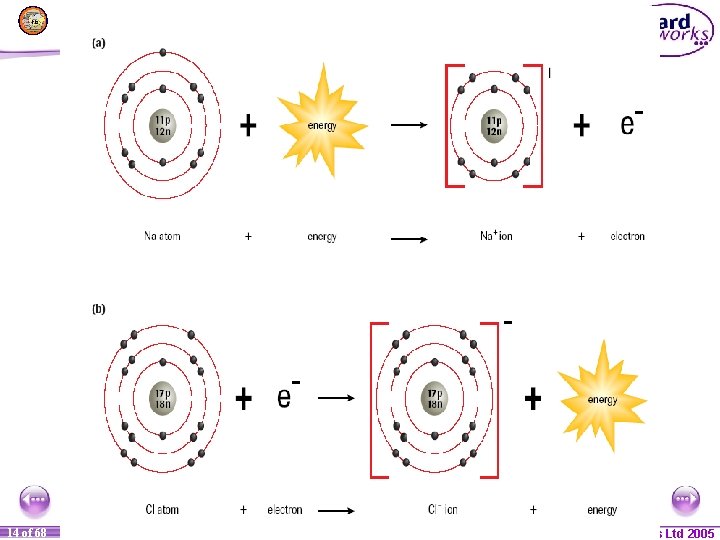
1 14 of of 20 68 © Boardworks Ltd 2005 2004
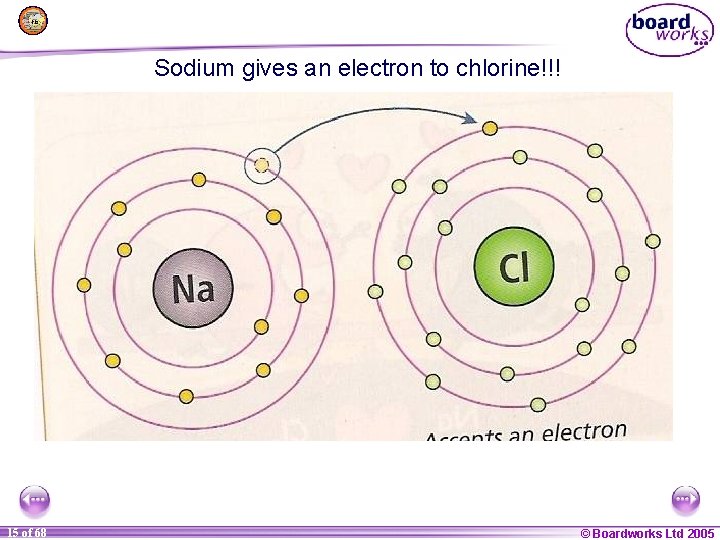
Sodium gives an electron to chlorine!!! 1 15 of of 20 68 © Boardworks Ltd 2005 2004
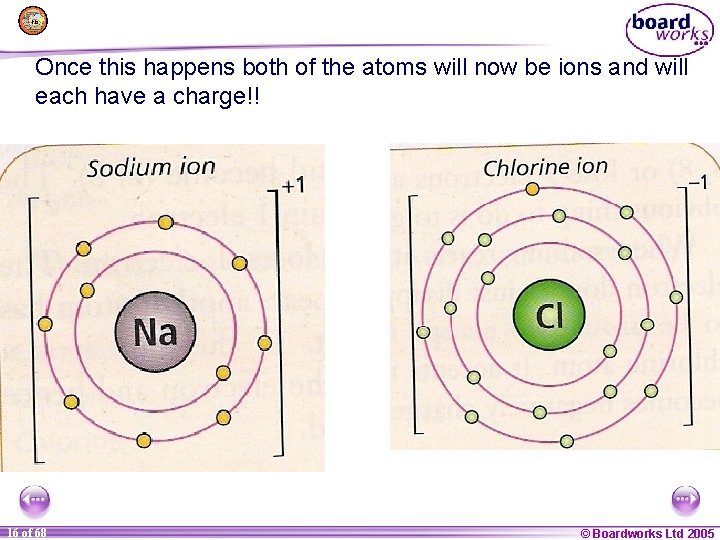
Once this happens both of the atoms will now be ions and will each have a charge!! 1 16 of of 20 68 © Boardworks Ltd 2005 2004

The oppositely charged ions attract each other and form an ionic bond!! 1 17 of of 20 68 © Boardworks Ltd 2005 2004

1 18 of of 20 68 © Boardworks Ltd 2005 2004
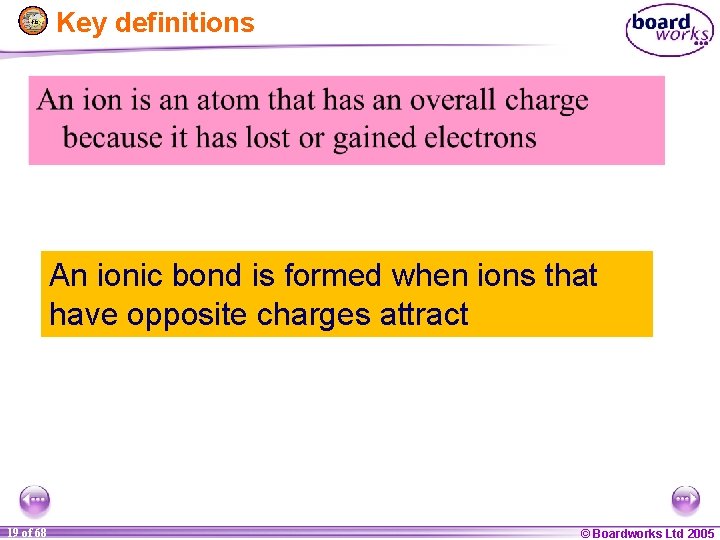
Key definitions An ionic bond is formed when ions that have opposite charges attract 1 19 of of 20 68 © Boardworks Ltd 2005 2004
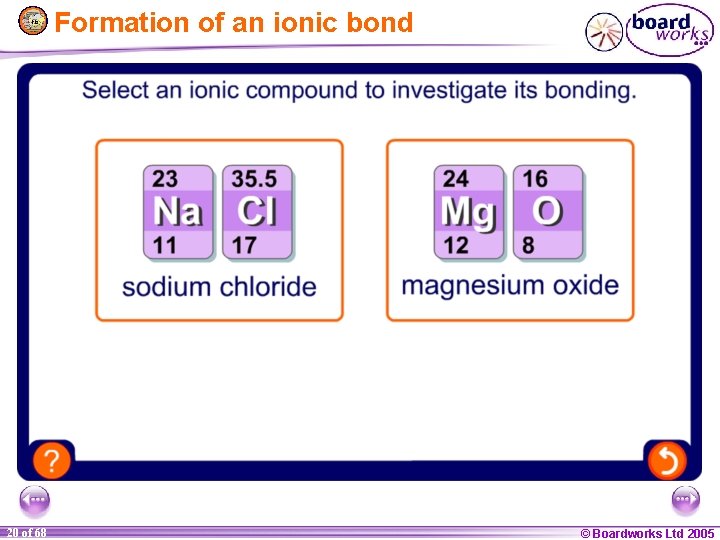
Formation of an ionic bond 1 20 of of 20 68 © Boardworks Ltd 2005 2004
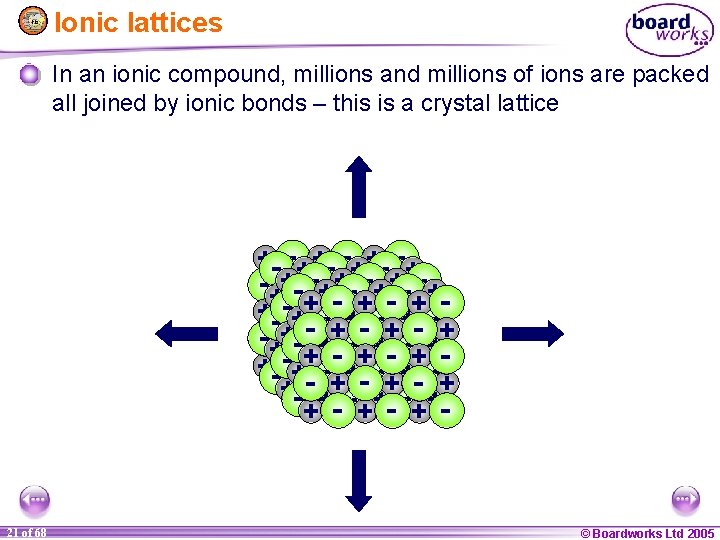
Ionic lattices In an ionic compound, millions and millions of ions are packed all joined by ionic bonds – this is a crystal lattice + ++ +++ +++ ++ ++ ++++ ++ + +++ ++ + -- -- -- - --- - -- - - - --- - -- -- -- 1 21 of of 20 68 © Boardworks Ltd 2005 2004

Bonding example 2 • Making Magnesium oxide 1 22 of of 20 68 © Boardworks Ltd 2005 2004
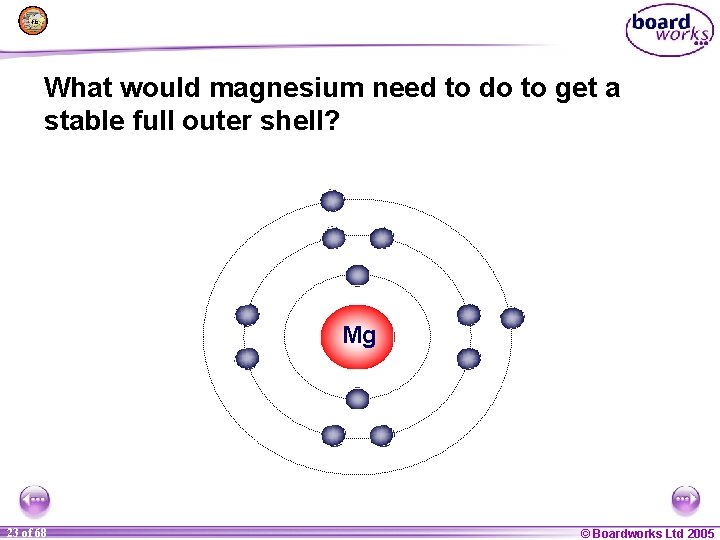
What would magnesium need to do to get a stable full outer shell? Mg 1 23 of of 20 68 © Boardworks Ltd 2005 2004
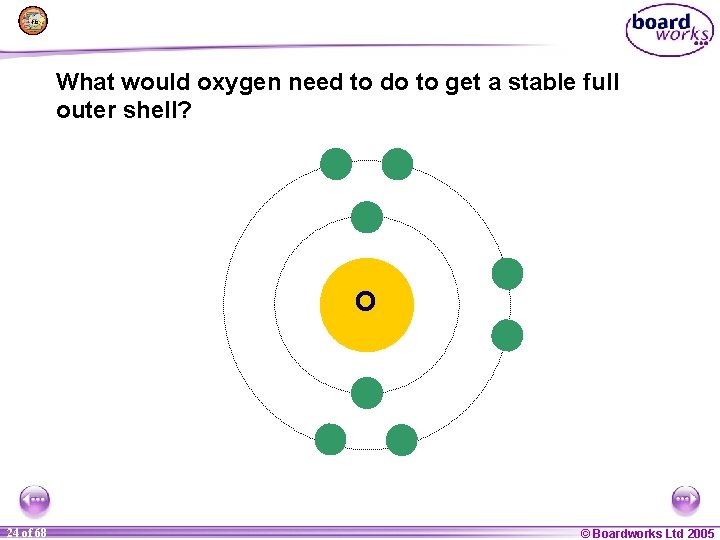
What would oxygen need to do to get a stable full outer shell? O 1 24 of of 20 68 © Boardworks Ltd 2005 2004
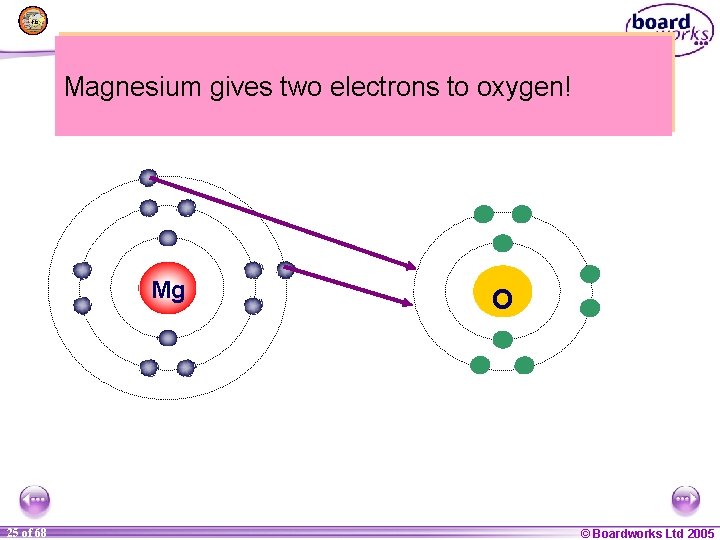
Can you think of a way that Magnesium could lose two electrons and two oxygen could to gain two Magnesium gives electrons oxygen! electrons? Mg 1 25 of of 20 68 O © Boardworks Ltd 2005 2004
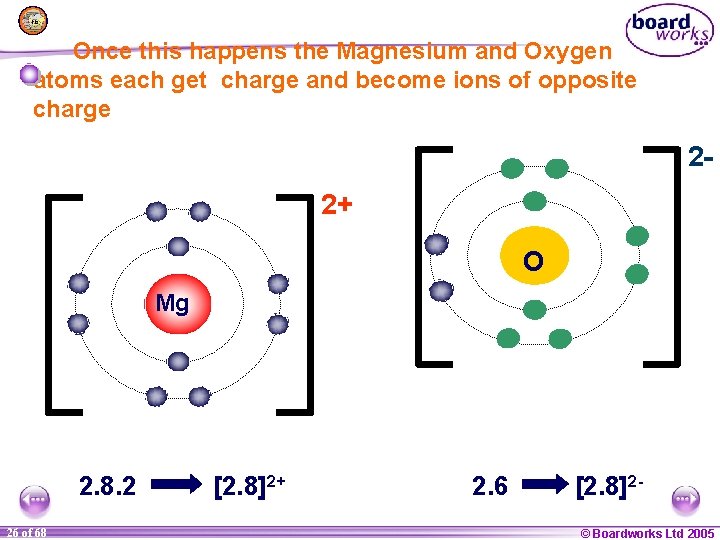
Once this happens the Magnesium and Oxygen atoms each get charge and become ions of opposite charge 22+ O Mg 2. 8. 2 1 26 of of 20 68 [2. 8]2+ 2. 6 [2. 8]2© Boardworks Ltd 2005 2004
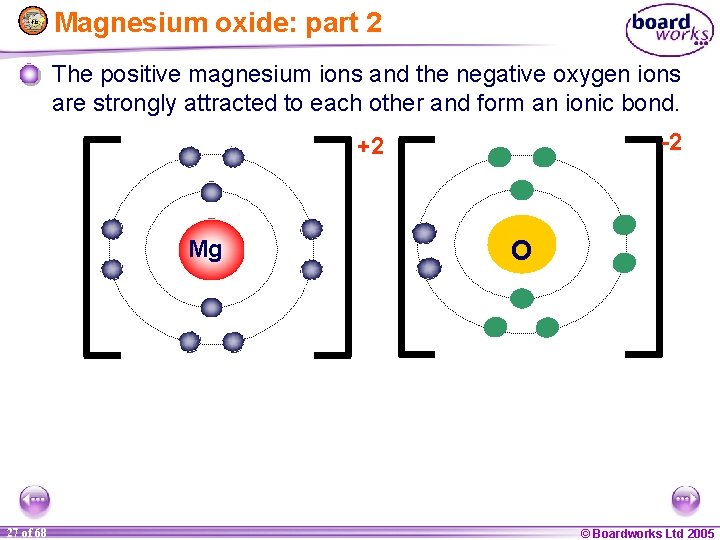
Magnesium oxide: part 2 The positive magnesium ions and the negative oxygen ions are strongly attracted to each other and form an ionic bond. -2 +2 Mg 1 27 of of 20 68 O © Boardworks Ltd 2005 2004
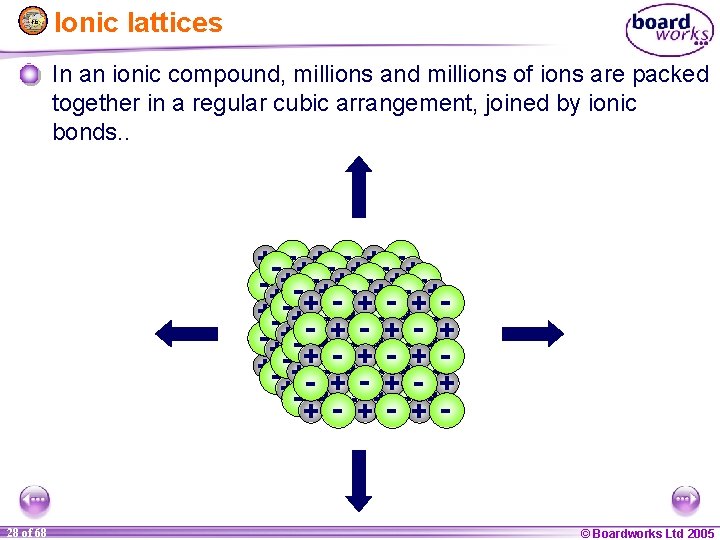
Ionic lattices In an ionic compound, millions and millions of ions are packed together in a regular cubic arrangement, joined by ionic bonds. . + ++ +++ +++ ++ ++ ++++ ++ + +++ ++ + -- -- -- - --- - -- - - - --- - -- -- -- 1 28 of of 20 68 © Boardworks Ltd 2005 2004
Formation of an ionic bond 1 29 of of 20 68 © Boardworks Ltd 2005 2004
MONOTOMIC IONS FORMING IONIC BONDS Example #1: React the following atoms to form an ionic bond Ca and Br Example #2: React the following atoms to form an ionic bond Al and O 1 30 of of 20 68 © Boardworks Ltd 2005 2004
Multiple-choice quiz 1 31 of of 20 68 © Boardworks Ltd 2005 2004
- Slides: 31