2 1 Classifying Matter Chapter 2 Section 1






































- Slides: 39

2. 1 Classifying Matter Chapter 2 Section 1

2. 1 Classifying Matter Each piece of your clothing has a label that recommends cleaning methods. A 100%-cotton shirt may need to be ironed after washing. A cotton and polyester blend fabric may come out of the dryer wrinkle free. There is no cleaning process that works for all materials.

2. 1 Classifying Matter Pure Substances Why are elements and compounds classified as pure substances?

2. 1 Classifying Matter Pure Substances A pure substance is matter that always has exactly the same composition. • Table salt and table sugar are two examples of pure substances. • Substances can be classified into two categories —elements and compounds.

2. 1 Classifying Matter Pure Substances Every sample of a given substance has the same properties because a substance has a fixed, uniform composition.

2. 1 Classifying Matter Elements An element is a substance that cannot be broken down into simpler substances. An element has a fixed composition because it contains only one type of atom. No two elements contain the same type of atom.

2. 1 Classifying Matter Elements Examples of Elements • Some elements are solids at room temperature. Most soft drink cans are made from aluminum. Carbon is the main element in the marks you make with a pencil. • The elements oxygen and nitrogen are the main gases in the air you breathe. • Two elements are liquids at room temperature–bromine and mercury.

2. 1 Classifying Matter Elements Symbols for Elements Chemists use symbols of one or two letters to represent elements. The first letter is always capitalized. If there is a second letter, it is not capitalized. • C represents carbon. • Al represents aluminum. • Au represents gold. (The Latin name for gold is aurum. )

2. 1 Classifying Matter Elements Symbols for Elements Symbols allow scientists who speak different languages to communicate without confusion. For example, nitrogen is azote in France, stickstoff in Germany, and nitrógeno in Mexico. But all scientists use N as the symbol for the element nitrogen.

2. 1 Classifying Matter Elements Aluminum, carbon, and gold are elements that you can see in common objects, such as cans, pencils, and rings. Mixtures containing iodine are used to prevent and treat infections. Gold Aluminum Carbon Iodine

2. 1 Classifying Matter Compounds A compound is a substance that is made from two or more simpler substances and can be broken down into those simpler substances. A compound always contains two or more elements joined in a fixed proportion.

2. 1 Classifying Matter Compounds The properties of a compound differ from those of the substances from which it is made. • Water is composed of the elements hydrogen and oxygen. Oxygen and hydrogen are gases at room temperature, but water is a liquid. • Hydrogen can fuel a fire, and oxygen can keep a fire burning, but water does not burn or help other substances to burn.

2. 1 Classifying Matter Compounds Silicon dioxide is a compound found in most lightcolored grains of sand in crystals of quartz. It is a colorless, transparent solid. Yet, silicon dioxide is made from a colorless gas (oxygen) and a gray solid (silicon). In silicon dioxide, there always two oxygen atoms for each silicon atom. Oxygen Silicon dioxide

2. 1 Classifying Matter Mixtures How do mixtures differ from pure substances?

2. 1 Classifying Matter Mixtures If you make salsa, a recipe can guide you. You can use exactly the amounts in the recipe, or you can adjust the ingredients to your own taste. Salsa is a mixture. Each batch may be slightly different.

2. 1 Classifying Matter Mixtures The properties of a mixture can vary because the composition of a mixture is not fixed. • Mixtures can retain some of the properties of their individual substances. • The properties of a mixture are less constant than the properties of a substance.

2. 1 Classifying Matter Mixtures can be classified by how well the parts of the mixture are distributed throughout the mixture. Heterogeneous Mixtures In a heterogeneous mixture, the parts of the mixture are noticeably different from one another. Homogeneous Mixtures In a homogeneous mixture, the substances are so evenly distributed that it is difficult to distinguish one substance in the mixture from another.

2. 1 Classifying Matter Mixtures The sand is a heterogeneous mixture of different kinds of grains. The spoon is stainless steel, a homogeneous mixture of iron, chromium, and nickel.

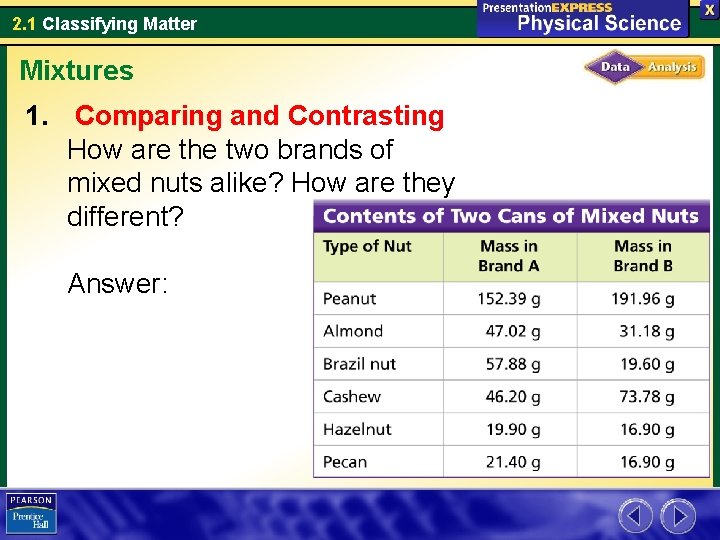
2. 1 Classifying Matter Mixtures Do the Contents of Two Cans of Mixed Nuts Meet FDA Regulations? According to Food and Drug Administration (FDA) regulations, a can labeled mixed nuts must contain at least four types of shelled nuts other than peanuts. The mass of each type of nut must be not less than 2 percent of the total mass and not more than 80 percent of the total mass.

2. 1 Classifying Matter Mixtures 1. Comparing and Contrasting How are the two brands of mixed nuts alike? How are they different? Answer:

2. 1 Classifying Matter Mixtures 1. Comparing and Contrasting How are the two brands of mixed nuts alike? How are they different? Answer: Both brands contain the same types of nuts but the amount of each type differs.

2. 1 Classifying Matter Mixtures 3. Drawing Conclusions Do the contents of each can meet the FDA regulations? Explain. Answer:

2. 1 Classifying Matter Mixtures 4. Inferring On the Brand A label, the nuts are listed in this order: peanuts, Brazil nuts, almonds, cashews, pecans, and hazelnuts. What do you think determines the order? Answer:

2. 1 Classifying Matter Solutions, Suspensions, and Colloids What is the main difference among solutions, suspensions, and colloids? The size of the particles in a mixture has an effect on the properties of the mixture. Based on the size of its largest particles, a mixture can be classified as a solution, a suspension, or a colloid.

2. 1 Classifying Matter Solutions, Suspensions, and Colloids Solutions When substances dissolve and form a homogeneous mixture, the mixture that forms is called a solution. Suspensions A suspension is a heterogeneous mixture that separates into layers over time.

2. 1 Classifying Matter Solutions, Suspensions, and Colloids A colloid contains some particles that are intermediate in size between the small particles in a solution and the larger particles in a suspension. • Like solutions, colloids do not separate into layers. • You cannot use a filter to separate the parts of a colloid.

2. 1 Classifying Matter Solutions, Suspensions, and Colloids These liquids represent three categories of mixtures. • Windshield wiper fluid is a solution. • Muddy water collected from a swamp is a suspension. • Milk is a colloid.

2. 1 Classifying Matter Assessment Questions 1. Which of these substances is a compound? a. b. c. d. copper water oxygen carbon

2. 1 Classifying Matter Assessment Questions 1. Which of these substances is a compound? a. b. c. d. copper water oxygen carbon ANS: B

2. 1 Classifying Matter Assessment Questions 2. Which of these groups of letters could be used as a symbol for an element? a. b. c. d. HF Cm Car fe

2. 1 Classifying Matter Assessment Questions 2. Which of these groups of letters could be used as a symbol for an element? a. b. c. d. HF Cm Car fe ANS: B

2. 1 Classifying Matter Assessment Questions 3. Which of the following statements does not apply to a compound? a. It is made of two or more elements. b. It has components that are joined in fixed proportions. c. It can be separated into components by physical methods. d. It can be broken down into elements or other compounds.

2. 1 Classifying Matter Assessment Questions 3. Which of the following statements does not apply to a compound? a. It is made of two or more elements. b. It has components that are joined in fixed proportions. c. It can be separated into components by physical methods. d. It can be broken down into elements or other compounds. ANS: C

2. 1 Classifying Matter Assessment Questions 4. How does a compound differ from a mixture? a. A compound cannot be broken down into simpler substances. b. Compounds can be separated by physical processes and mixtures cannot. c. The composition of a mixture cannot vary. d. A compound is made of two or more elements in fixed proportion.

2. 1 Classifying Matter Assessment Questions 4. How does a compound differ from a mixture? a. A compound cannot be broken down into simpler substances. b. Compounds can be separated by physical processes and mixtures cannot. c. The composition of a mixture cannot vary. d. A compound is made of two or more elements in fixed proportion. ANS: D

2. 1 Classifying Matter Assessment Questions 5. Which of these materials is a heterogeneous mixture? a. b. c. d. air seawater sand steel

2. 1 Classifying Matter Assessment Questions 5. Which of these materials is a heterogeneous mixture? a. b. c. d. air seawater sand steel ANS: C

2. 1 Classifying Matter Assessment Questions 6. Which of the following can be separated with a filter? a. b. c. d. colloids compounds solutions suspensions

2. 1 Classifying Matter Assessment Questions 6. Which of the following can be separated with a filter? a. b. c. d. colloids compounds solutions suspensions ANS: D