1990 2000 2004 6 2004 9 2004 10




















- Slides: 32


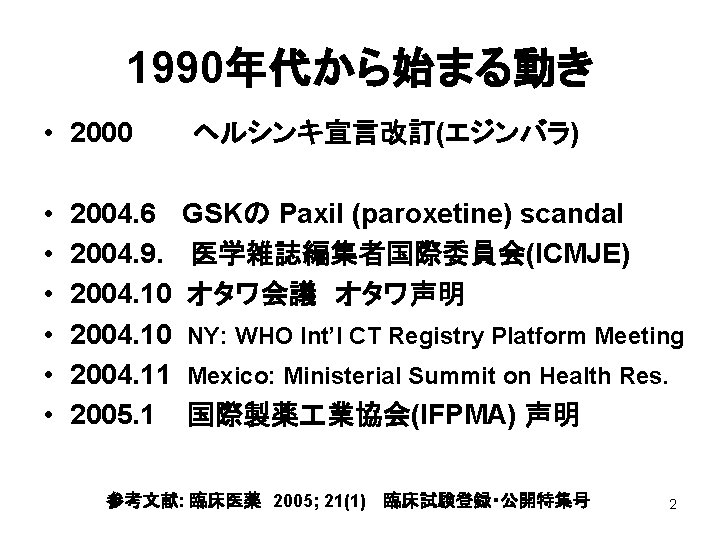
1990年代から始まる動き • 2000 • • • 2004. 6 2004. 9. 2004. 10 2004. 11 2005. 1 ヘルシンキ宣言改訂(エジンバラ) GSKの Paxil (paroxetine) scandal 医学雑誌編集者国際委員会(ICMJE) オタワ会議 オタワ声明 NY: WHO Int’l CT Registry Platform Meeting Mexico: Ministerial Summit on Health Res. 国際製薬 業協会(IFPMA) 声明 参考文献: 臨床医薬 2005; 21(1) 臨床試験登録・公開特集号 2

Statement of NY Workgroup (Oct. 2004) • Need for global approach to clinical trials registration – – – Unambiguous identification of trials Consensus needed on which trials; data; timing and disclosure of results One-stop search portal; publicly available System that is simple, effective, efficient Capacity built where appropriate • WHO should establish formal process toward a global approach – – Appropriate governance Collaborative process, involving all interested parties Existing structures leveraged; need for any new structures identified WHO mindful of ICMJE deadline 3

NY会議でまとめられた 10項目 1. Why register / disclose 2. Which trials to register 3. When to register 4. What data - Unique ID 5. What data – Trial descriptors 6. When to update 7. What to disclose at completion 8. Register characteristics 9. Ensuring compliance 10. Role for WHO 4 General agreement / Area requiring further discussion


6

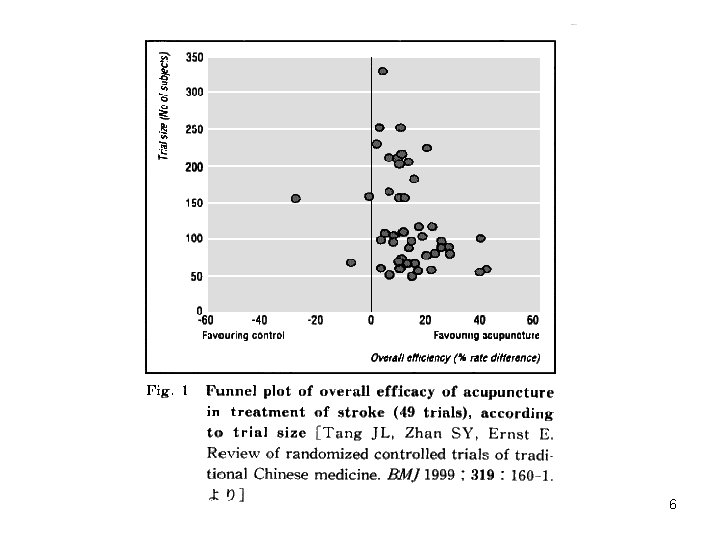
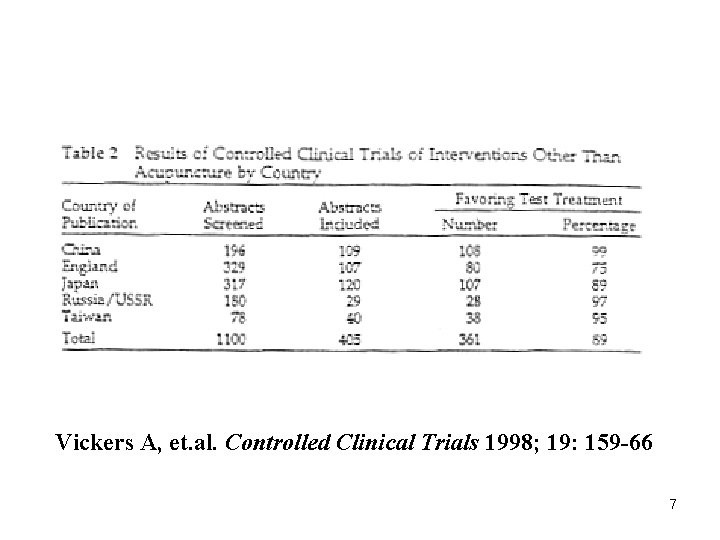
Vickers A, et. al. Controlled Clinical Trials 1998; 19: 159 -66 7



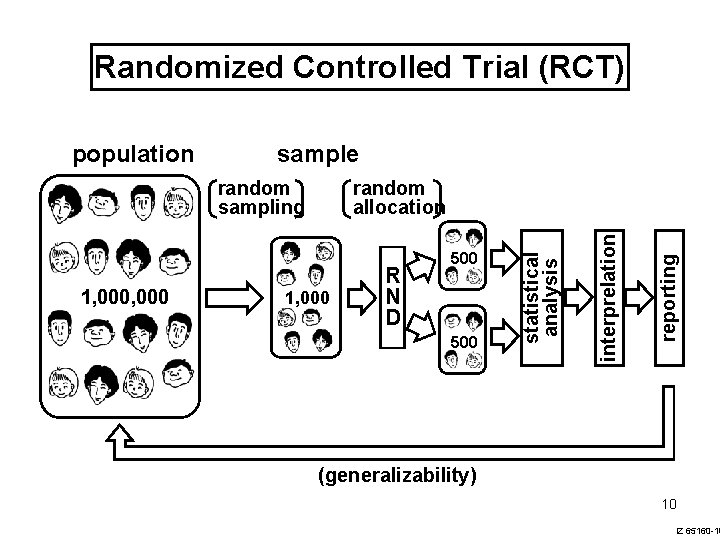
Randomized Controlled Trial (RCT) sample 1, 000 R N D 500 reporting 1, 000 random allocation interprelation random sampling statistical analysis population (generalizability) 10 IZ 65160 -10


ベルモントレポート(1979) 1. Respect for person 人の尊重 オートノミー 2. Beneficence 善行 3. Non-maleficence 無危害 4. Justice 正義 12



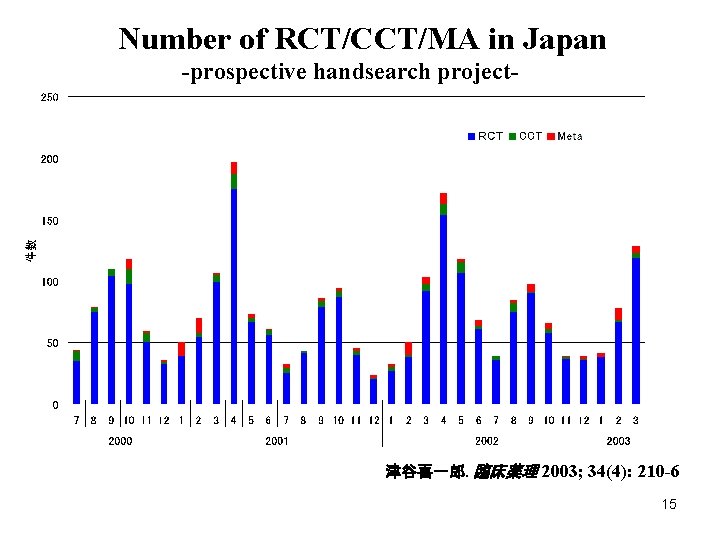
Number of RCT/CCT/MA in Japan -prospective handsearch project- 津谷喜一郎. 臨床薬理 2003; 34(4): 210 -6 15

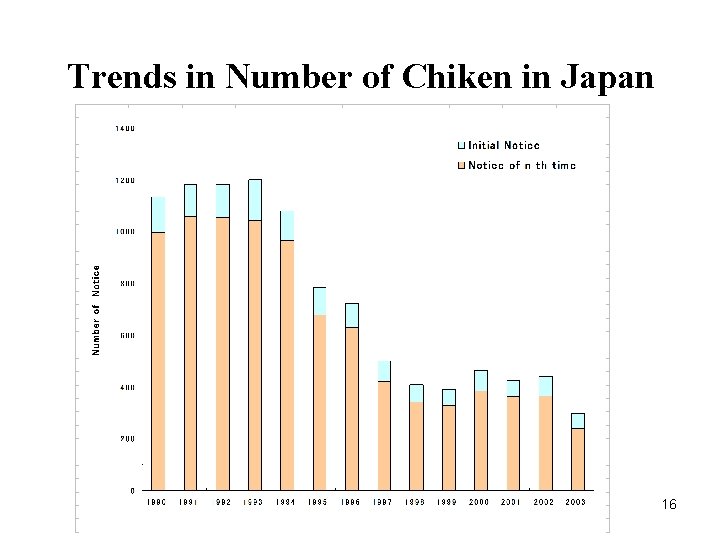
Trends in Number of Chiken in Japan 16

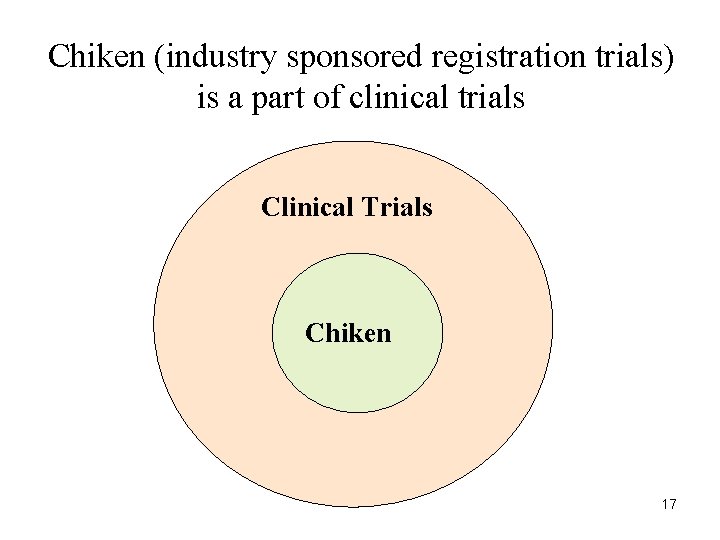
Chiken (industry sponsored registration trials) is a part of clinical trials Clinical Trials Chiken 17

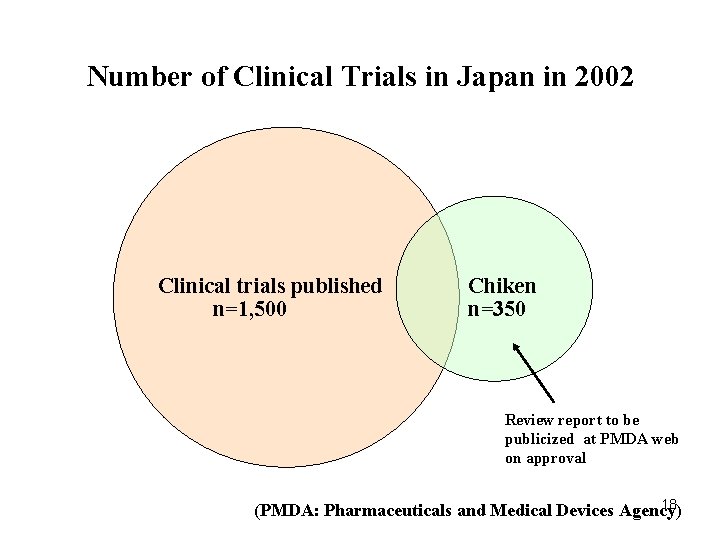
Number of Clinical Trials in Japan in 2002 Clinical trials published n=1, 500 Chiken n=350 Review report to be publicized at PMDA web on approval 18 (PMDA: Pharmaceuticals and Medical Devices Agency)

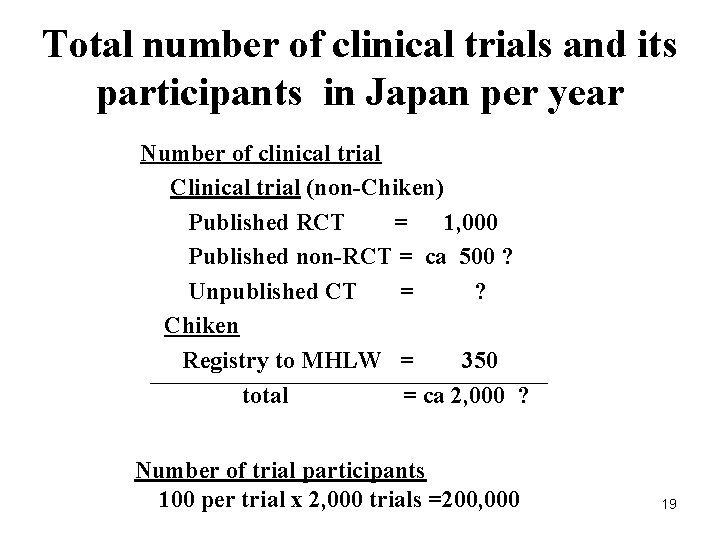
Total number of clinical trials and its participants in Japan per year Number of clinical trial Clinical trial (non-Chiken) Published RCT = 1, 000 Published non-RCT = ca 500 ? Unpublished CT = ? Chiken Registry to MHLW = 350 total = ca 2, 000 ? Number of trial participants 100 per trial x 2, 000 trials =200, 000 19









10. WHOの役割 • Provide facilitation standards developing area capacity • Avoid duplication bureaucracy Fund ? 28

朝日新聞 2004. 7. 13 29


