19 2 Amino Acids as Acids and Bases
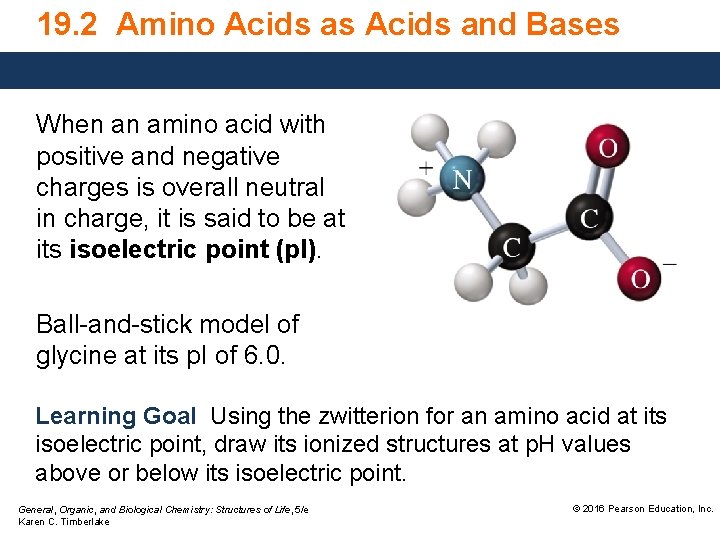
19. 2 Amino Acids as Acids and Bases When an amino acid with positive and negative charges is overall neutral in charge, it is said to be at its isoelectric point (p. I). Ball-and-stick model of glycine at its p. I of 6. 0. Learning Goal Using the zwitterion for an amino acid at its isoelectric point, draw its ionized structures at p. H values above or below its isoelectric point. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
Isoelectric Point The isoelectric point of an amino acid is the p. H at which • the charged groups on an amino acid are balanced. • the amino acid is neutral. An amino acid can exist as • a positive ion if a solution is more acidic (lower p. H) than its p. I. • a negative ion if a solution is more basic (higher p. H) than its p. I. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Ionized Forms of Amino Acids The p. I values for nonpolar and polar neutral amino acids are from p. H 5. 1 to 6. 3. Alanine has a zero overall charge at its p. I of 6. 0 with a carboxylate anion (— COO−) and an ammonium cation (—NH 3+). General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Ionized Forms of Amino Acids Alanine adds an H+ to the carboxyl group (— COO−) when the solution is more acidic than its p. I (p. H < 6). General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
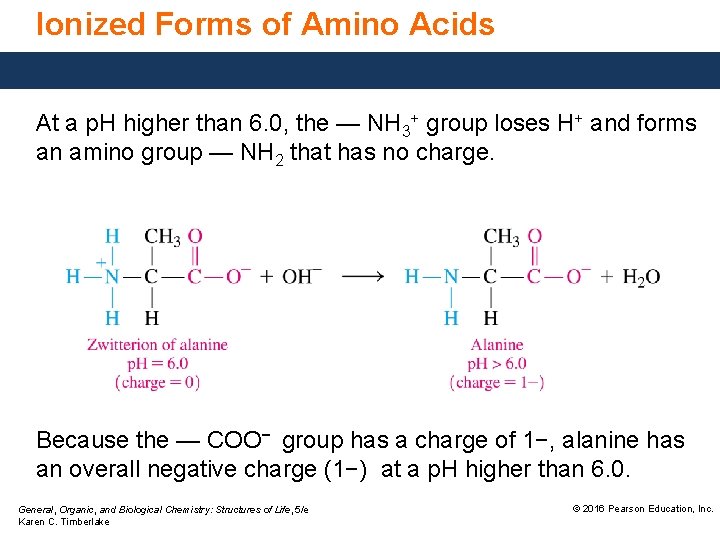
Ionized Forms of Amino Acids At a p. H higher than 6. 0, the — NH 3+ group loses H+ and forms an amino group — NH 2 that has no charge. Because the — COO− group has a charge of 1−, alanine has an overall negative charge (1−) at a p. H higher than 6. 0. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
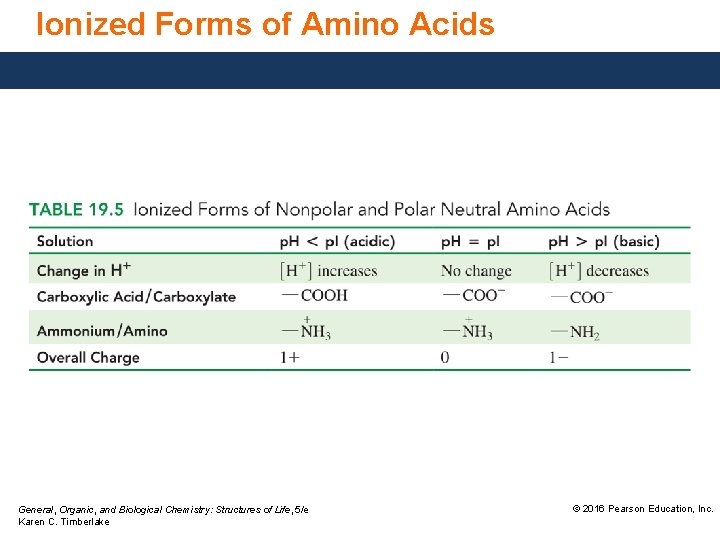
Ionized Forms of Amino Acids General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Ionized Forms of Polar Acidic and Polar Basic Amino Acids General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
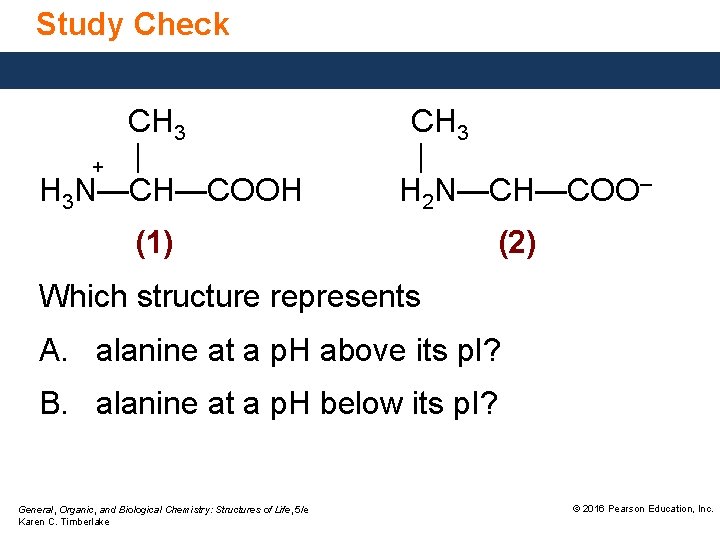
Study Check CH 3 + | H 3 N—CH—COOH CH 3 | H 2 N—CH—COO– (1) (2) Which structure represents A. alanine at a p. H above its p. I? B. alanine at a p. H below its p. I? General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
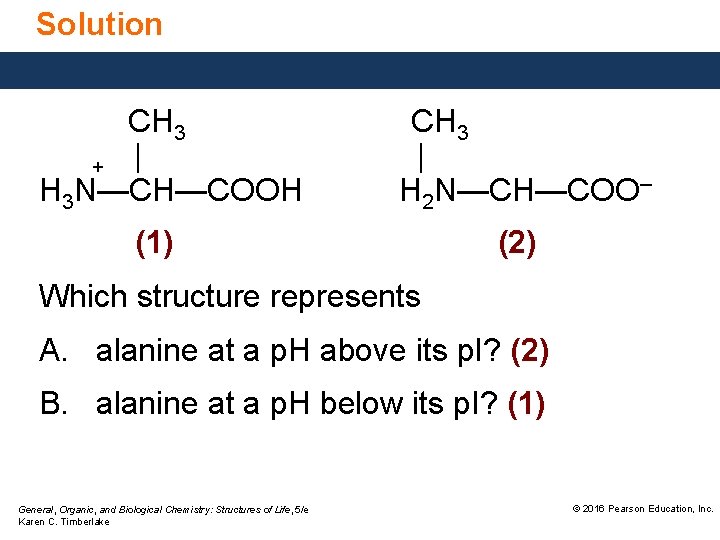
Solution CH 3 + | H 3 N—CH—COOH CH 3 | H 2 N—CH—COO– (1) (2) Which structure represents A. alanine at a p. H above its p. I? (2) B. alanine at a p. H below its p. I? (1) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
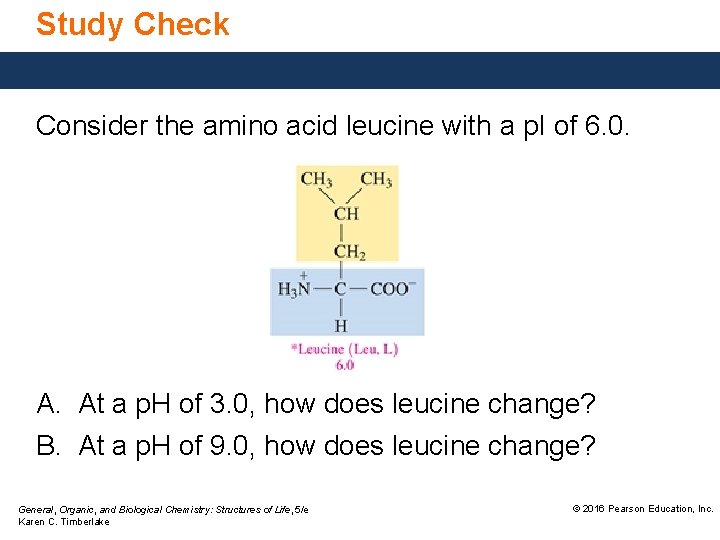
Study Check Consider the amino acid leucine with a p. I of 6. 0. A. At a p. H of 3. 0, how does leucine change? B. At a p. H of 9. 0, how does leucine change? General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
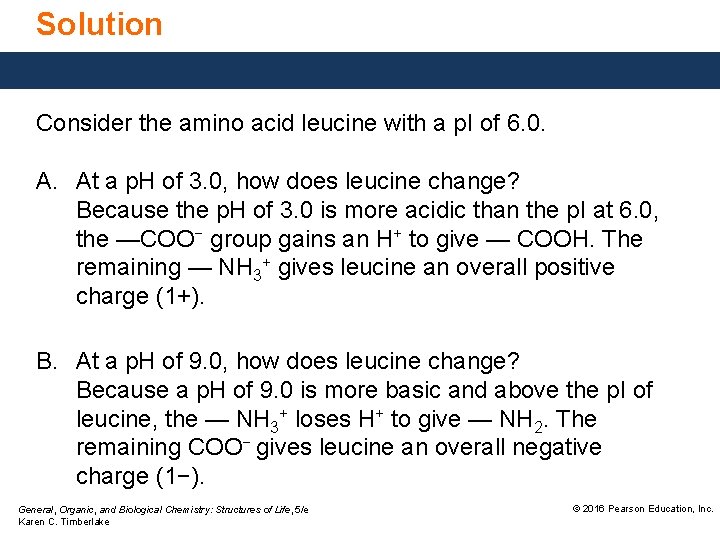
Solution Consider the amino acid leucine with a p. I of 6. 0. A. At a p. H of 3. 0, how does leucine change? Because the p. H of 3. 0 is more acidic than the p. I at 6. 0, the —COO− group gains an H+ to give — COOH. The remaining — NH 3+ gives leucine an overall positive charge (1+). B. At a p. H of 9. 0, how does leucine change? Because a p. H of 9. 0 is more basic and above the p. I of leucine, the — NH 3+ loses H+ to give — NH 2. The remaining COO− gives leucine an overall negative charge (1−). General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
- Slides: 11