18 29 Consider the decomposition of calcium carbonate
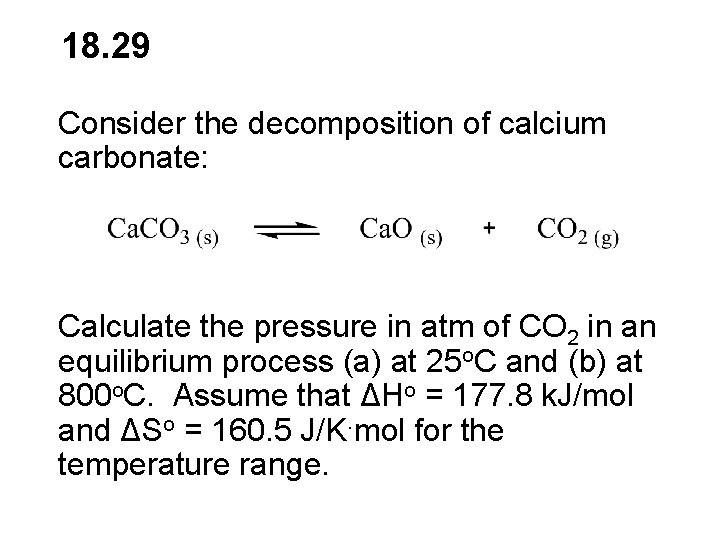
18. 29 Consider the decomposition of calcium carbonate: Calculate the pressure in atm of CO 2 in an equilibrium process (a) at 25 o. C and (b) at 800 o. C. Assume that ΔHo = 177. 8 k. J/mol and ΔSo = 160. 5 J/K. mol for the temperature range.
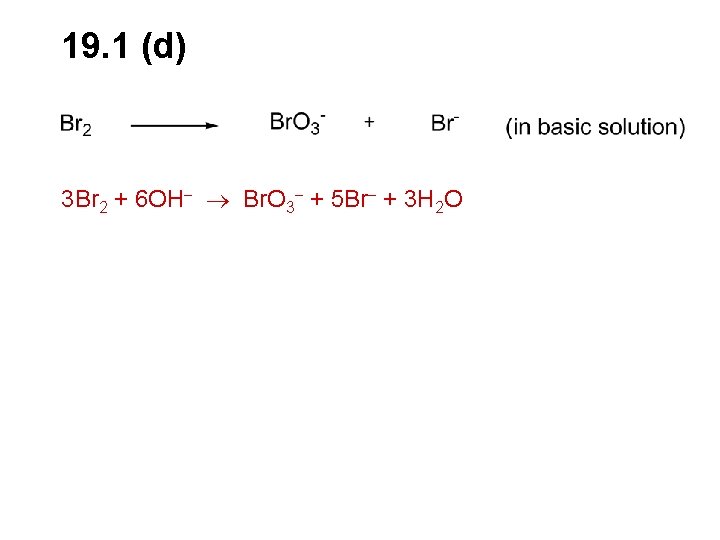
19. 1 (d) 3 Br 2 + 6 OH Br. O 3 + 5 Br + 3 H 2 O

Example 14. 4 • What is the rate constant for a first order reaction that converts 74% of starting material to product in 33 minutes? 0. 0408 min-1 • What is the half-life of this reaction? 17 min
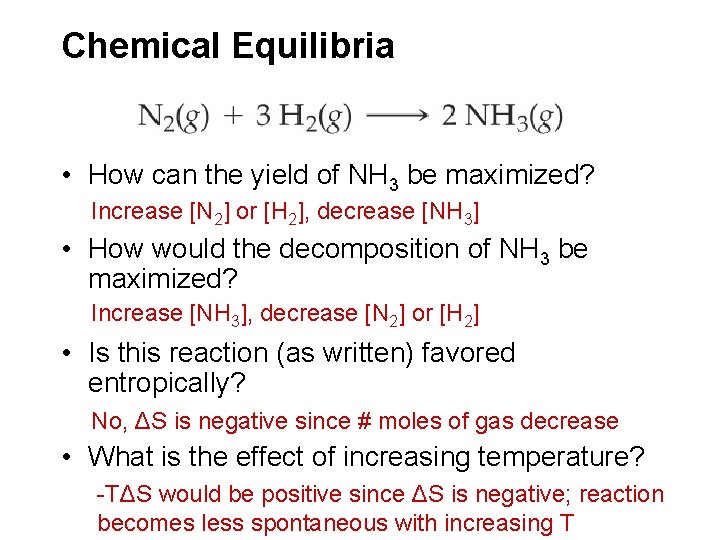
Chemical Equilibria • How can the yield of NH 3 be maximized? Increase [N 2] or [H 2], decrease [NH 3] • How would the decomposition of NH 3 be maximized? Increase [NH 3], decrease [N 2] or [H 2] • Is this reaction (as written) favored entropically? No, ΔS is negative since # moles of gas decrease • What is the effect of increasing temperature? -TΔS would be positive since ΔS is negative; reaction becomes less spontaneous with increasing T
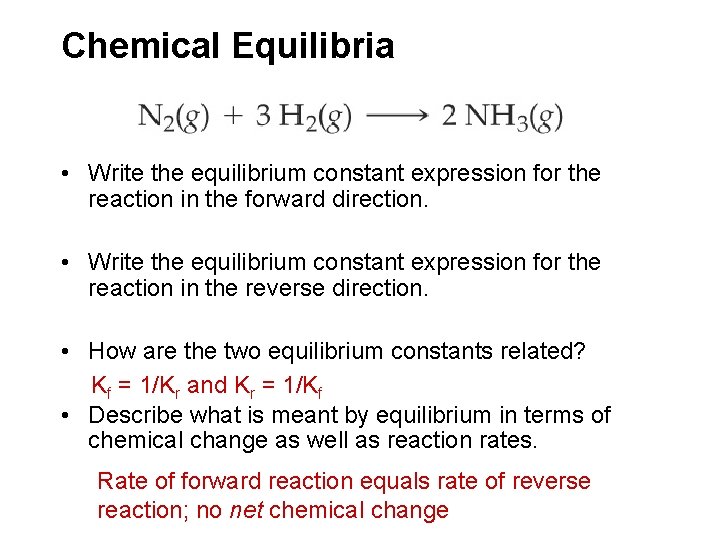
Chemical Equilibria • Write the equilibrium constant expression for the reaction in the forward direction. • Write the equilibrium constant expression for the reaction in the reverse direction. • How are the two equilibrium constants related? Kf = 1/Kr and Kr = 1/Kf • Describe what is meant by equilibrium in terms of chemical change as well as reaction rates. Rate of forward reaction equals rate of reverse reaction; no net chemical change
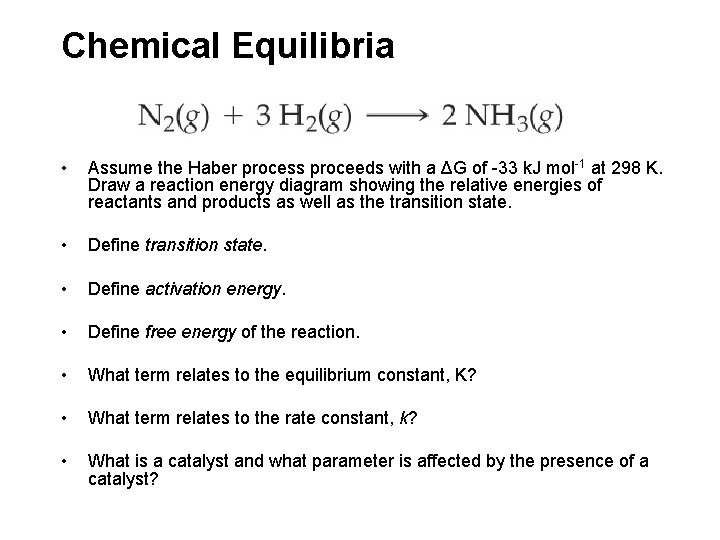
Chemical Equilibria • Assume the Haber process proceeds with a ΔG of -33 k. J mol-1 at 298 K. Draw a reaction energy diagram showing the relative energies of reactants and products as well as the transition state. • Define activation energy. • Define free energy of the reaction. • What term relates to the equilibrium constant, K? • What term relates to the rate constant, k? • What is a catalyst and what parameter is affected by the presence of a catalyst?

Colligative properties: freezing point depression • Calculate the approximate freezing point of a solution made from 21. 0 g Na. Cl and 1. 00 × 102 g of H 2 O. Kf of water is 1. 86°C/m. A. B. C. D. E. 3. 59°C 6. 68°C -13. 4°C -6. 68°C -3. 59°C The molality would be 2 X (van’t Hoff factor, i = 2)

Colligative properties: freezing point depression • Calculate the approximate freezing point of a solution made from 21. 0 g Na. Cl and 1. 00 × 102 g of H 2 O. Kf of water is 1. 86°C/m. • How would having 21. 0 g of ethanol (CH 3 CH 2 OH) affect the calculation? The molality would be 1 X, i = 1) • How would the calculation be affected if the salt were KNO 3? The molality would be 2 X, i = 2)
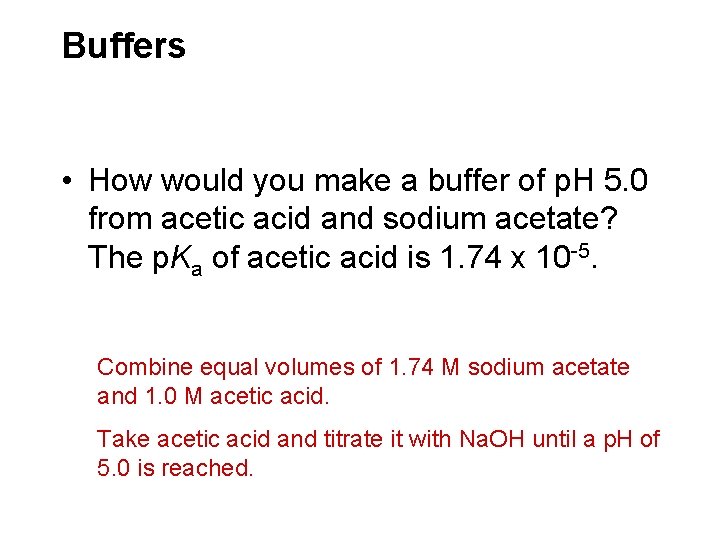
Buffers • How would you make a buffer of p. H 5. 0 from acetic acid and sodium acetate? The p. Ka of acetic acid is 1. 74 x 10 -5. Combine equal volumes of 1. 74 M sodium acetate and 1. 0 M acetic acid. Take acetic acid and titrate it with Na. OH until a p. H of 5. 0 is reached.
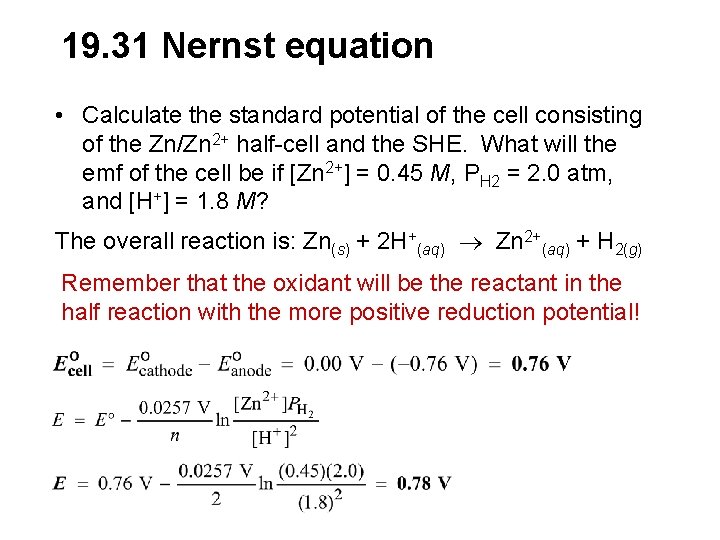
19. 31 Nernst equation • Calculate the standard potential of the cell consisting of the Zn/Zn 2+ half-cell and the SHE. What will the emf of the cell be if [Zn 2+] = 0. 45 M, PH 2 = 2. 0 atm, and [H+] = 1. 8 M? The overall reaction is: Zn(s) + 2 H+(aq) Zn 2+(aq) + H 2(g) Remember that the oxidant will be the reactant in the half reaction with the more positive reduction potential!
- Slides: 10