17 7 8 Electrolysis Applications Since chemical oxidationreduction













- Slides: 26

17. 7 -8 Electrolysis & Applications • Since chemical oxidation-reduction involves the transfer of electrons from one substance to another, it should be possible to harness the flow of electrons to produce electricity. We do this with voltaic cells. • Electricity can also be used to cause nonspontaneous chemical reactions (i. e. recharging batteries). This process is called electrolysis (carried out in electrolytic cells) 1

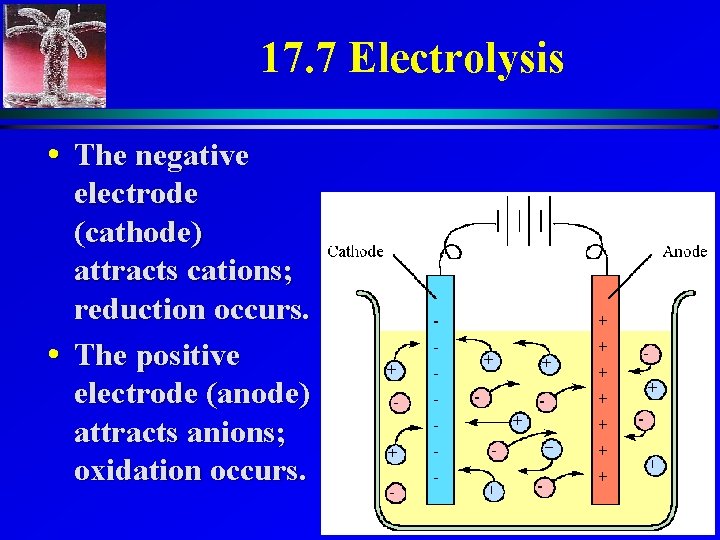
17. 7 Electrolysis • Electrolysis is used for isolating active elements, purifying metals, and electroplating. • Pure compounds: H 2 O, molten salts • Use inert electrodes in the liquid and pass electricity through the system 3

17. 7 Electrolysis • The negative electrode (cathode) attracts cations; reduction occurs. • The positive electrode (anode) attracts anions; oxidation occurs. 4

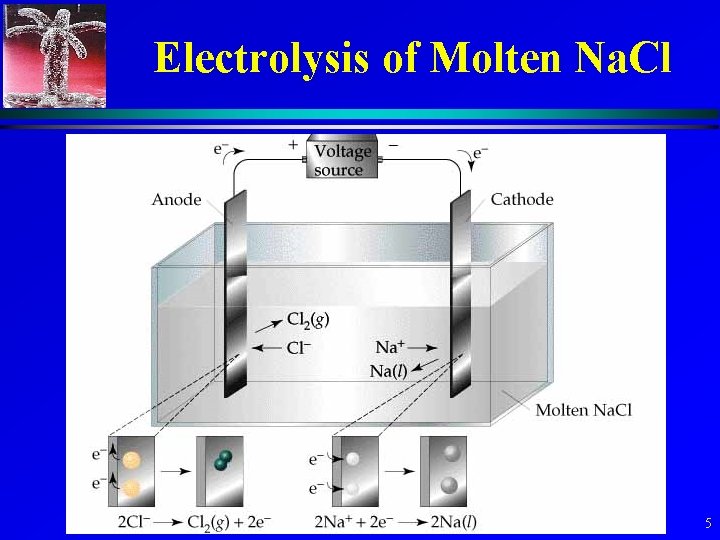
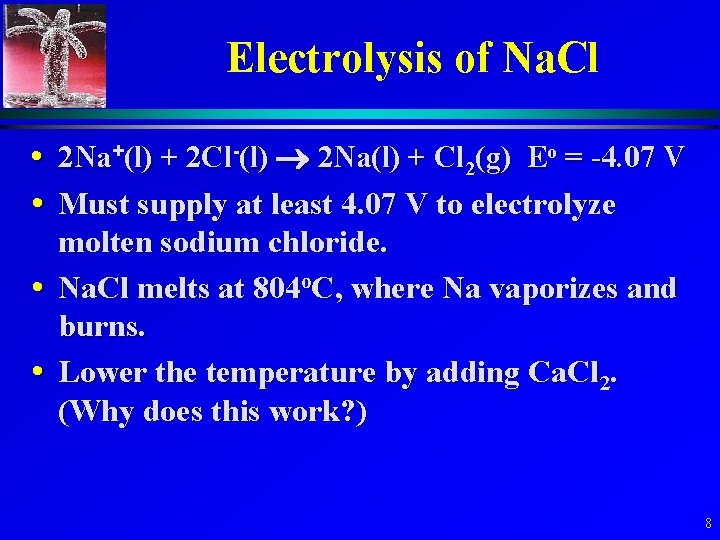
Electrolysis of Molten Na. Cl 5

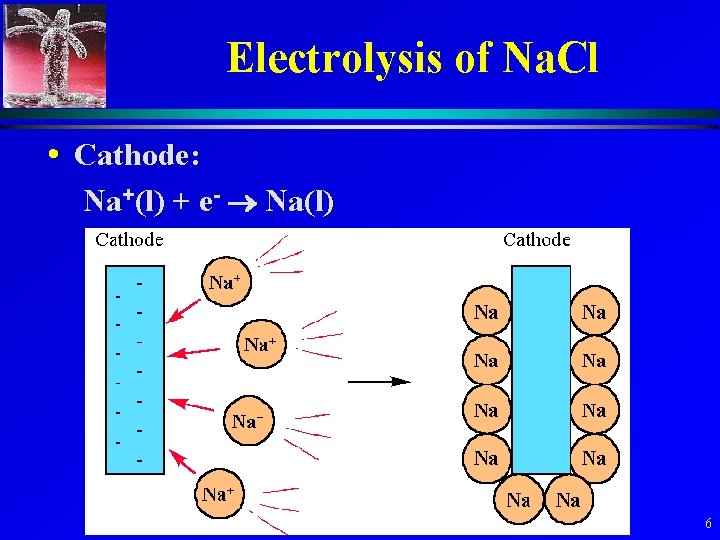
Electrolysis of Na. Cl • Cathode: Na+(l) + e- Na(l) 6

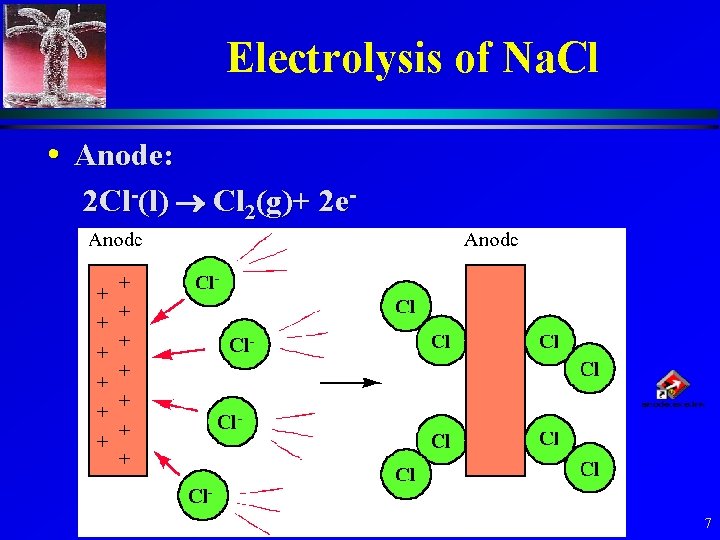
Electrolysis of Na. Cl • Anode: 2 Cl-(l) Cl 2(g)+ 2 e- 7

Electrolysis of Na. Cl • 2 Na+(l) + 2 Cl-(l) 2 Na(l) + Cl 2(g) Eo = -4. 07 V • Must supply at least 4. 07 V to electrolyze molten sodium chloride. • Na. Cl melts at 804 o. C, where Na vaporizes and burns. • Lower the temperature by adding Ca. Cl 2. (Why does this work? ) 8

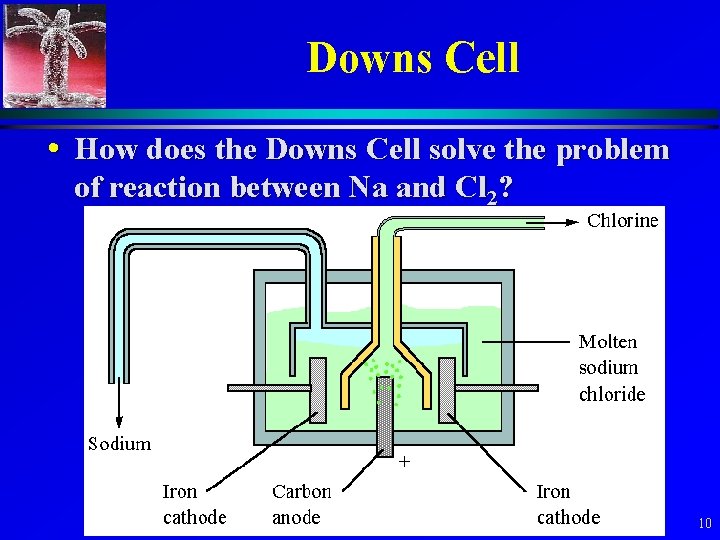
Electrolysis of Na. Cl • Other problem, Na reacts with Cl 2, even at room temperature. • Commercial operations use a Downs Cell. (described in 17. 8, pg 859) 9

Downs Cell • How does the Downs Cell solve the problem of reaction between Na and Cl 2? 10

Applications of Electrolysis • Electrolysis can be used in a variety of applications: • • Chemical recovery of elements in mixtures Industrial recovery of elements, mining Plating out of metals, electroplating. And many more! 11

Industrial Processes • Purification of Copper: Recovered from its ores by chemical reduction. • Purified by electrolysis. • Recover impurities: • • • Mo (25%) Se (93%) Te (96%) Au (32%) Ag (28%) 12

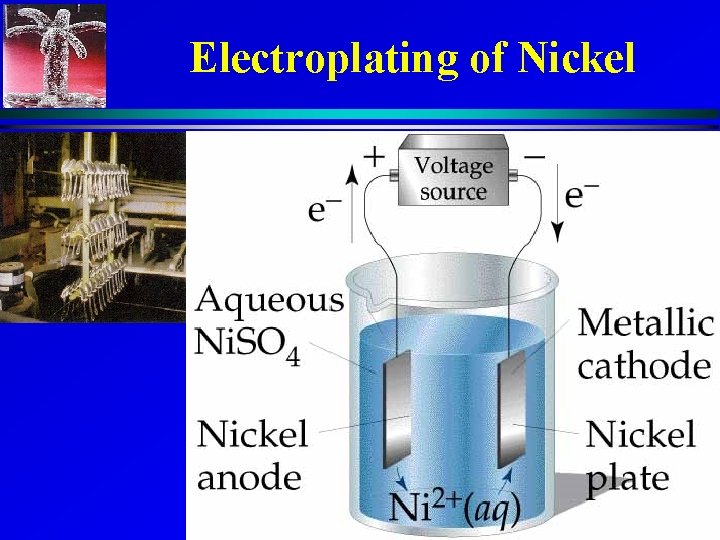
Electroplating of Nickel 13

Electrolytic Processes with Metals • A variety of metals can be prepared by electrolysis, if a cheap source of electricity is available. In addition, some metals* are purified by electrolysis. • • • aluminum calcium gold* magnesium zinc cadmium copper* lead* sodium 14

Faraday’s Law • Recall… • F = charge on 1 mol e- = 96500 coul/mol • and Electrical Current = charge / time • • 1 ampere = 1 coulomb of charge / second 1 A = 1 coul / s • Use these relationships to analyze electrolytic processes • 77 a, 79 a, 81 15

17. 7 Faraday’s Law • Faraday’s Law: the mass of product produced by a given amount of current is proportional to the number of electrons transferred. 16

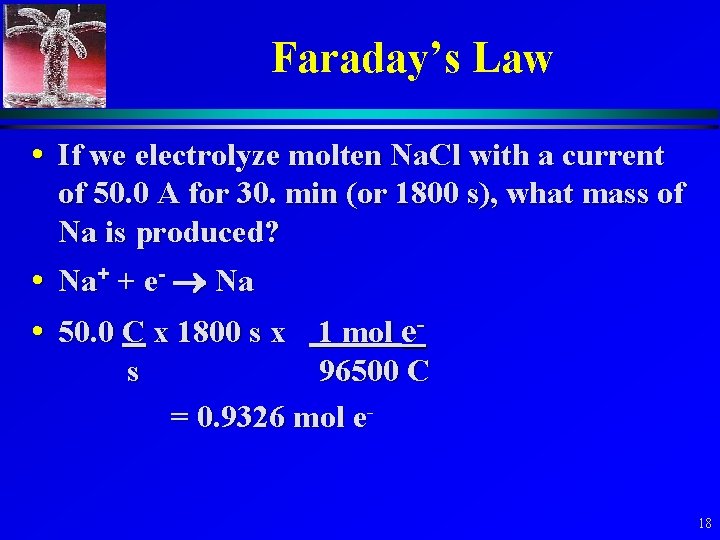
Faraday’s Law • If we electrolyze molten Na. Cl with a current of 50. 0 A for 30. min (or 1800 s), what mass of Na is produced? • Na+ + e- Na • 50. 0 C x 1800 s x 1 mol es 96500 C = 0. 9326 mol e 18

Faraday’s Law • moles Na = 0. 9326 mol e- x 1 mol Na/1 mol e= 0. 9326 mol • mass Na = 0. 9326 mol x 22. 99 g/mol = 21. 44 g • We can also calculate how much electrical energy it will take for an electrolysis. We will not pursue these calculations. 19

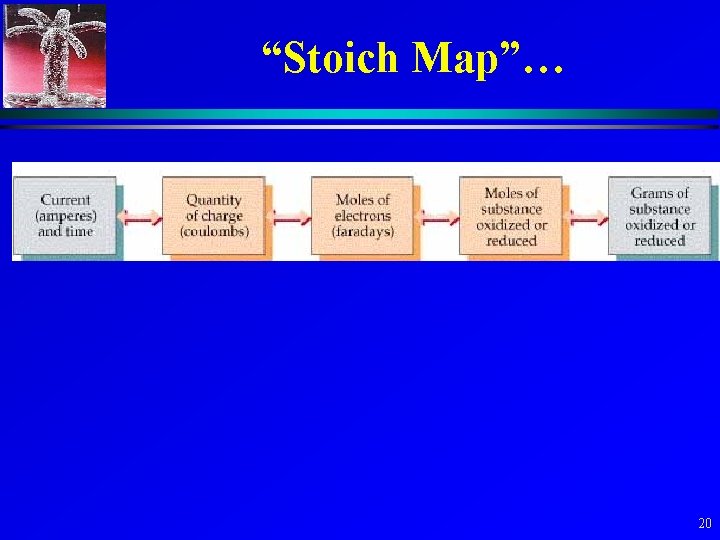
“Stoich Map”… 20

Revisit Electrolysis of KI(aq) Introduction 21

Electrolysis of H 2 O • Anode (oxidation): 2 H 2 O O 2(g) + 4 H+ + 4 e • Cathode (reduction): 2 H 2 O + 2 e- H 2(g) + 2 OH 2 H 2 O 2 H 2(g) + O 2(g) Eoxo = -1. 23 V Eredo = -0. 83 V Ecello = -2. 06 V • Must supply at least 2. 06 V to electrolyze water (if anode [H+] = 1. 0 M and cathode [OH-] = 1. 0 M) • In pure water, [H+] = [OH-] = 10 -7 M and the overall potential is – 1. 23 V • An electrolyte is usually added to increase electrical conductivity 24

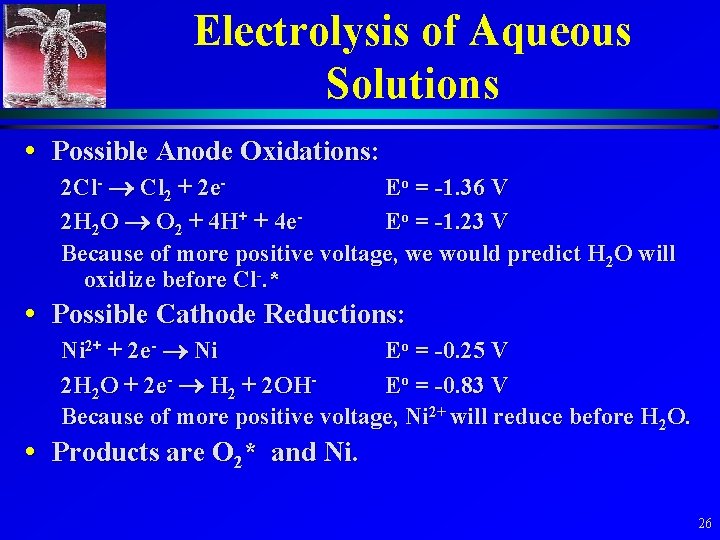
Electrolysis of Aqueous Solutions • Products depend on whether it is easier to oxidize or reduce the dissolved ions or water. • Sample Problem: Consider a solution of Ni. Cl 2 under standard conditions. 25

Electrolysis of Aqueous Solutions • Possible Anode Oxidations: 2 Cl- Cl 2 + 2 e. Eo = -1. 36 V 2 H 2 O O 2 + 4 H+ + 4 e. Eo = -1. 23 V Because of more positive voltage, we would predict H 2 O will oxidize before Cl-. * • Possible Cathode Reductions: Ni 2+ + 2 e- Ni Eo = -0. 25 V 2 H 2 O + 2 e- H 2 + 2 OHEo = -0. 83 V Because of more positive voltage, Ni 2+ will reduce before H 2 O. • Products are O 2* and Ni. 26

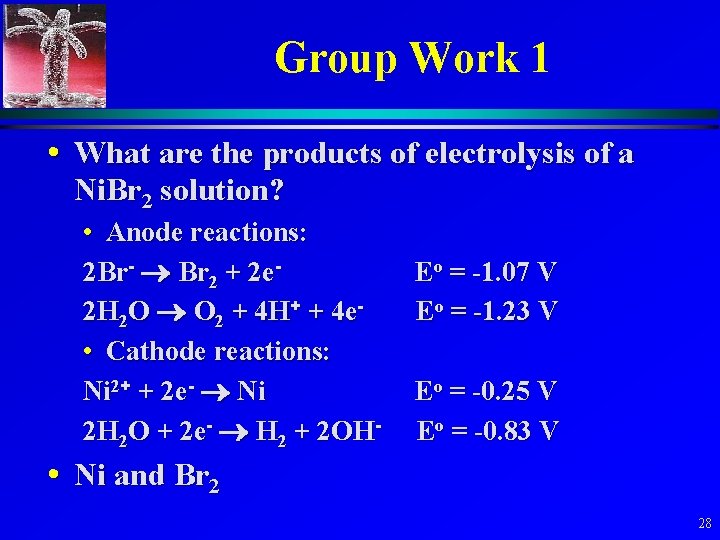
Group Work 1. What are the products of electrolysis of an aqueous Ni. Br 2 solution? 2. What are the products of electrolysis of an aqueous Cu. F 2 solution? 3. What are the products of electrolysis of a mixture of aqueous Cu. Br 2 and Ni. F 2? 27

Group Work 1 • What are the products of electrolysis of a Ni. Br 2 solution? • Anode reactions: 2 Br- Br 2 + 2 e 2 H 2 O O 2 + 4 H+ + 4 e • Cathode reactions: Ni 2+ + 2 e- Ni 2 H 2 O + 2 e- H 2 + 2 OH- Eo = -1. 07 V Eo = -1. 23 V Eo = -0. 25 V Eo = -0. 83 V • Ni and Br 2 28

Group Work 2 • What are the products of electrolysis of a Cu. F 2 aqueous solution? • Anode: 2 F- F 2 + 2 e 2 H 2 O O 2 + 4 H+ + 4 e- Eo = -2. 87 V Eo = -1. 23 V Cu 2+ + 2 e- Cu 2 H 2 O + 2 e- H 2 + 2 OH- Eo = 0. 34 V Eo = -0. 83 V • Cathode: • Cu and O 2 29

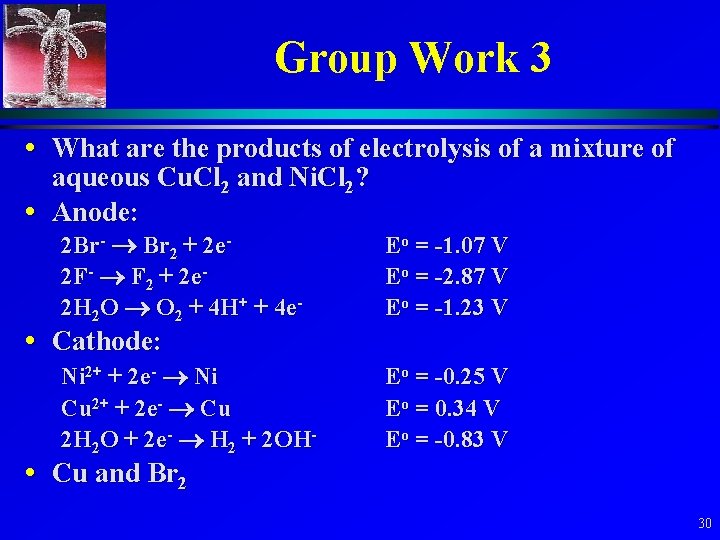
Group Work 3 • What are the products of electrolysis of a mixture of aqueous Cu. Cl 2 and Ni. Cl 2? • Anode: 2 Br- Br 2 + 2 e 2 F- F 2 + 2 e 2 H 2 O O 2 + 4 H+ + 4 e- Eo = -1. 07 V Eo = -2. 87 V Eo = -1. 23 V Ni 2+ + 2 e- Ni Cu 2+ + 2 e- Cu 2 H 2 O + 2 e- H 2 + 2 OH- Eo = -0. 25 V Eo = 0. 34 V Eo = -0. 83 V • Cathode: • Cu and Br 2 30