16 6 Polyprotic Acids Phosphoric acid A triprotic
- Slides: 15

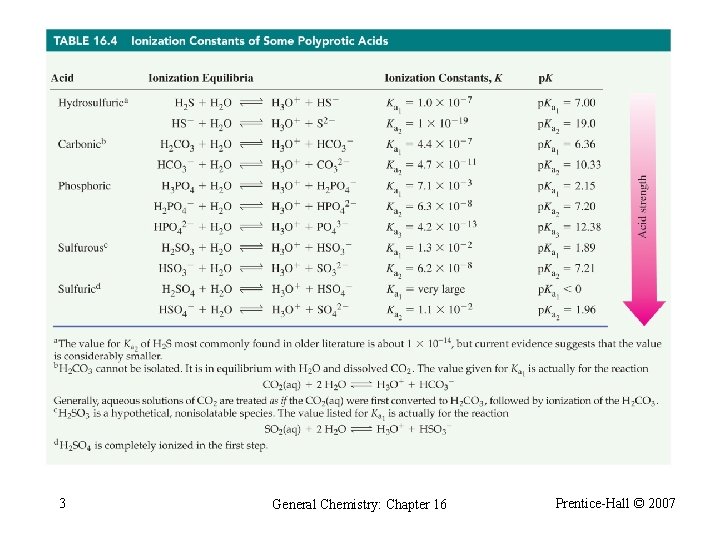
16 -6 Polyprotic Acids Phosphoric acid: A triprotic acid. 1 H 3 PO 4 + H 2 O H 3 O+ + H 2 PO 4 - Ka = 7. 1 10 -3 H 2 PO 4 - + H 2 O H 3 O+ + HPO 42 - Ka = 6. 3 10 -8 HPO 42 - + H 2 O H 3 O+ + PO 43 - Ka = 4. 2 10 -13 General Chemistry: Chapter 16 Prentice-Hall © 2007

Phosphoric Acid ¨ Ka 1 >> Ka 2 ◦ All H 3 O+ is formed in the first ionization step. ¨ H 2 PO 4 - essentially does not ionize further. ◦ Assume [H 2 PO 4 -] = [H 3 O+]. ¨ [HPO 42 -] Ka 2 regardless of solution molarity. 2 General Chemistry: Chapter 16 Prentice-Hall © 2007

3 General Chemistry: Chapter 16 Prentice-Hall © 2007

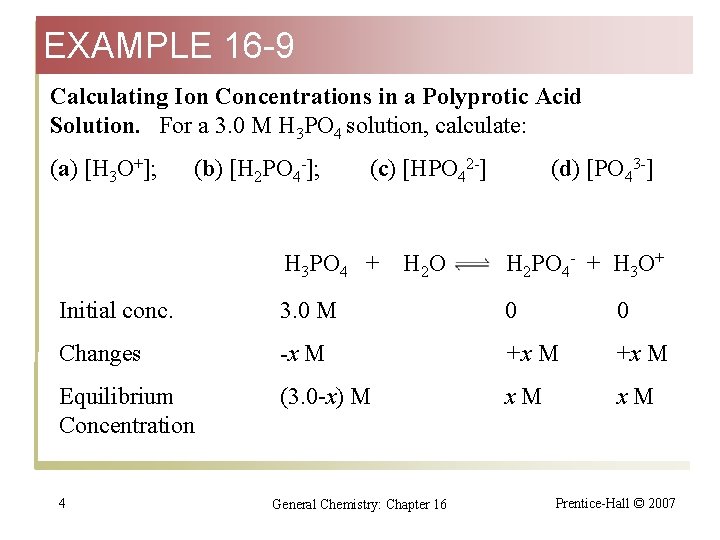
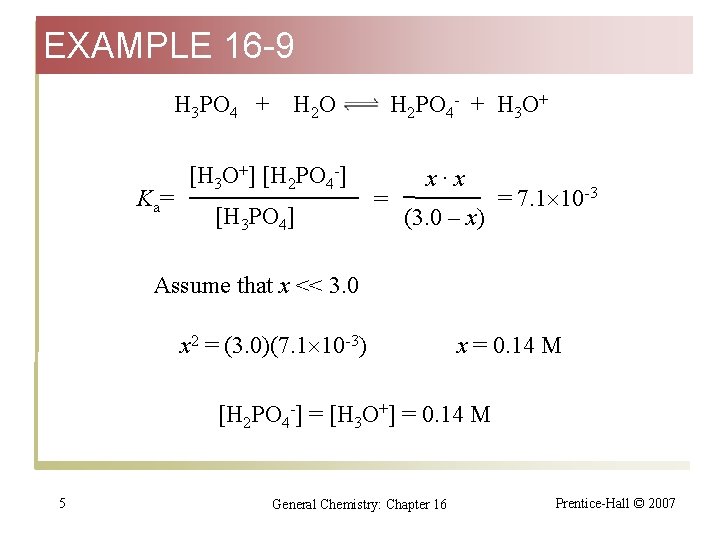
EXAMPLE 16 -9 Calculating Ion Concentrations in a Polyprotic Acid Solution. For a 3. 0 M H 3 PO 4 solution, calculate: (a) [H 3 O+]; (b) [H 2 PO 4 -]; (c) [HPO 42 -] H 3 PO 4 + H 2 O (d) [PO 43 -] H 2 PO 4 - + H 3 O+ Initial conc. 3. 0 M 0 0 Changes -x M +x M Equilibrium Concentration (3. 0 -x) M x. M 4 General Chemistry: Chapter 16 Prentice-Hall © 2007

EXAMPLE 16 -9 H 3 PO 4 + K a= H 2 O [H 3 O+] [H 2 PO 4 -] [H 3 PO 4] H 2 PO 4 - + H 3 O+ = x·x (3. 0 – x) = 7. 1 10 -3 Assume that x << 3. 0 x 2 = (3. 0)(7. 1 10 -3) x = 0. 14 M [H 2 PO 4 -] = [H 3 O+] = 0. 14 M 5 General Chemistry: Chapter 16 Prentice-Hall © 2007

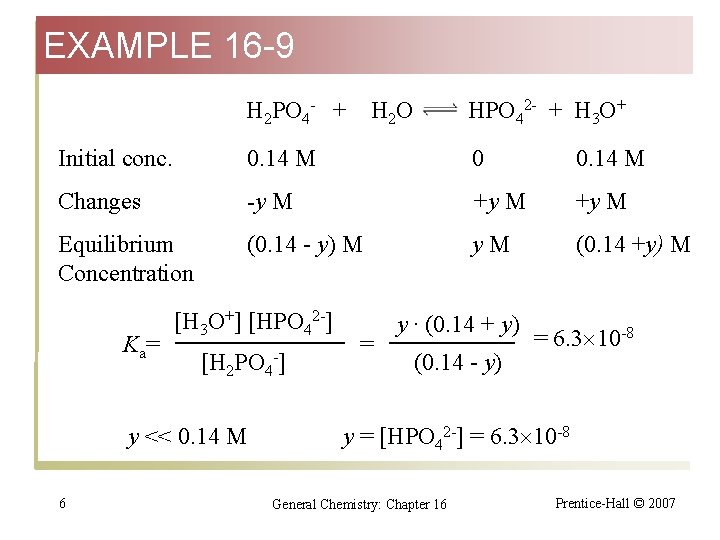
EXAMPLE 16 -9 H 2 PO 4 - + H 2 O HPO 42 - + H 3 O+ Initial conc. 0. 14 M 0 0. 14 M Changes -y M +y M Equilibrium Concentration (0. 14 - y) M y. M (0. 14 +y) M K a= [H 3 O+] [HPO 42 -] [H 2 PO 4 -] y << 0. 14 M 6 = y · (0. 14 + y) (0. 14 - y) = 6. 3 10 -8 y = [HPO 42 -] = 6. 3 10 -8 General Chemistry: Chapter 16 Prentice-Hall © 2007

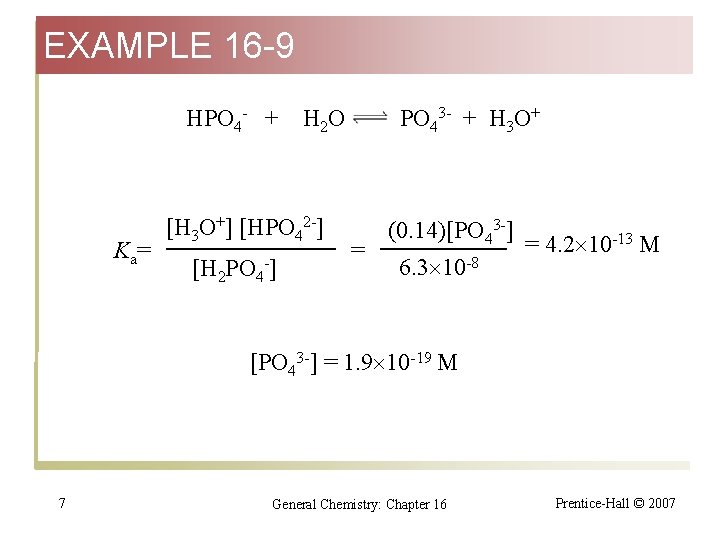
EXAMPLE 16 -9 HPO 4 - + K a= H 2 O [H 3 O+] [HPO 42 -] [H 2 PO 4 -] PO 43 - + H 3 O+ = (0. 14)[PO 43 -] 6. 3 10 -8 = 4. 2 10 -13 M [PO 43 -] = 1. 9 10 -19 M 7 General Chemistry: Chapter 16 Prentice-Hall © 2007

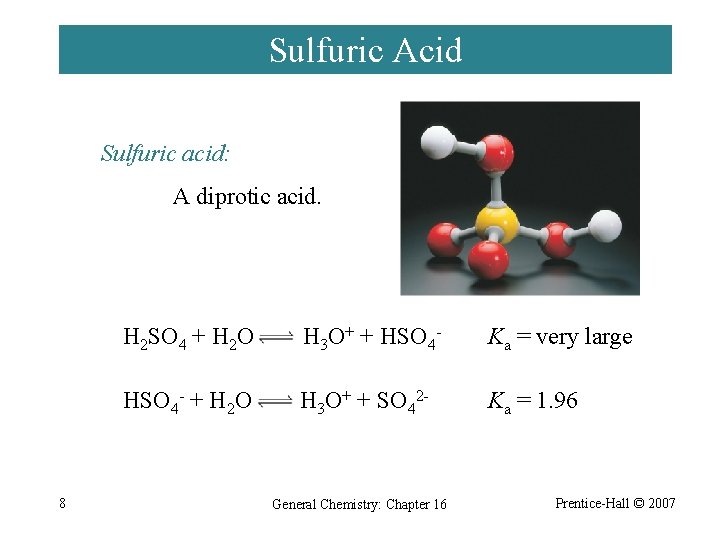
Sulfuric Acid Sulfuric acid: A diprotic acid. 8 H 2 SO 4 + H 2 O H 3 O+ + HSO 4 - Ka = very large HSO 4 - + H 2 O H 3 O+ + SO 42 - Ka = 1. 96 General Chemistry: Chapter 16 Prentice-Hall © 2007

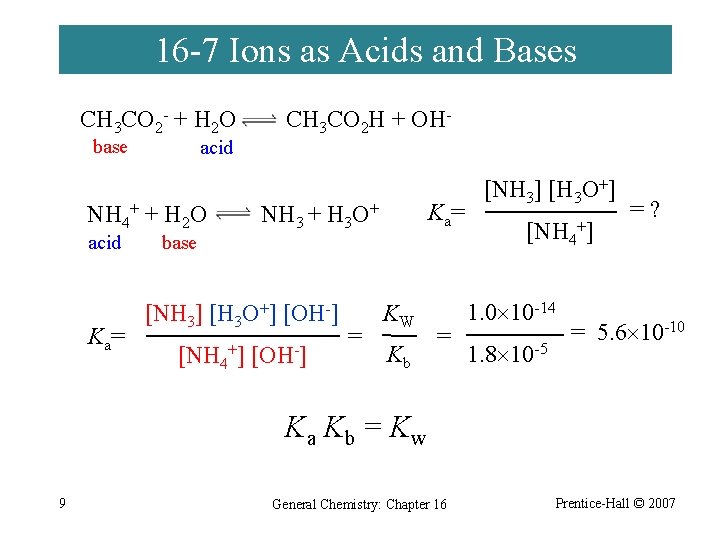
16 -7 Ions as Acids and Bases CH 3 CO 2 - + H 2 O base acid NH 4+ + H 2 O acid K a= base CH 3 CO 2 H + OH- K a= NH 3 + H 3 O+ [NH 3] [H 3 O+] [OH-] [NH 4+] [OH-] = KW Kb = [NH 3] [H 3 O+] [NH 4+] 1. 0 10 -14 1. 8 10 -5 =? = 5. 6 10 -10 Ka Kb = Kw 9 General Chemistry: Chapter 16 Prentice-Hall © 2007

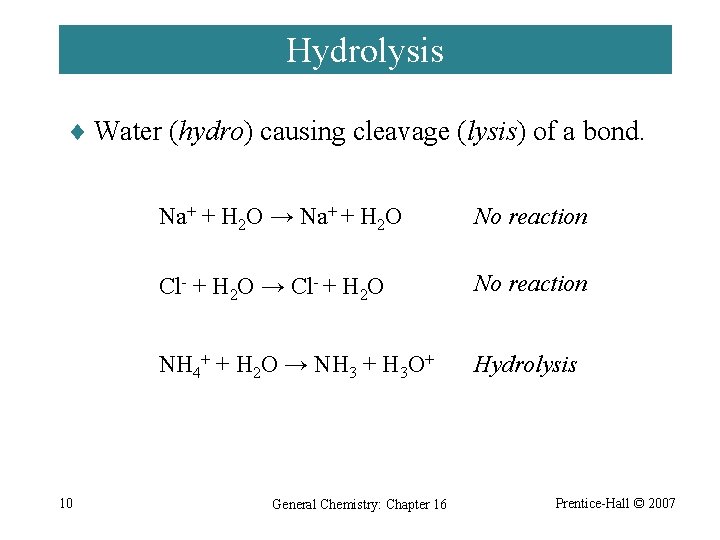
Hydrolysis ¨ Water (hydro) causing cleavage (lysis) of a bond. 10 Na+ + H 2 O → Na+ + H 2 O No reaction Cl- + H 2 O → Cl- + H 2 O No reaction NH 4+ + H 2 O → NH 3 + H 3 O+ Hydrolysis General Chemistry: Chapter 16 Prentice-Hall © 2007

16 -8 Molecular Structure and Acid-Base Behavior ¨ Why is CH 3 CO 2 H a stronger acid than CH 3 CH 2 OH? ¨ There is a relationship between molecular structure and acid strength. ¨ Bond dissociation energies are measured in the gas phase and not in solution. 11 General Chemistry: Chapter 16 Prentice-Hall © 2007

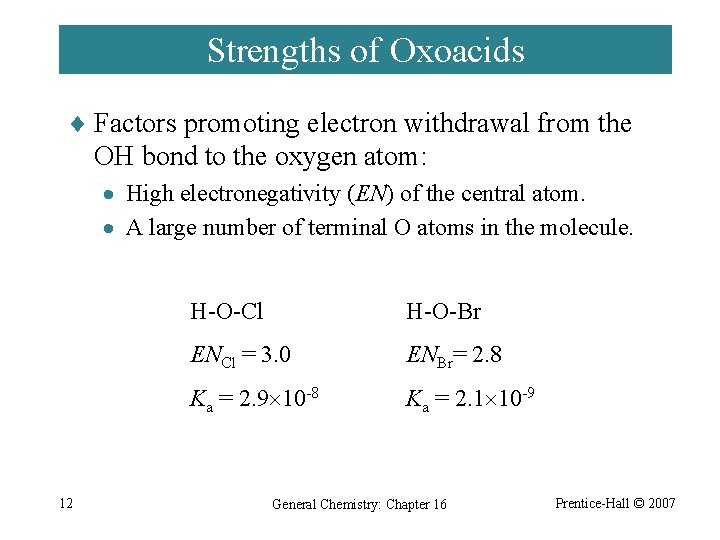
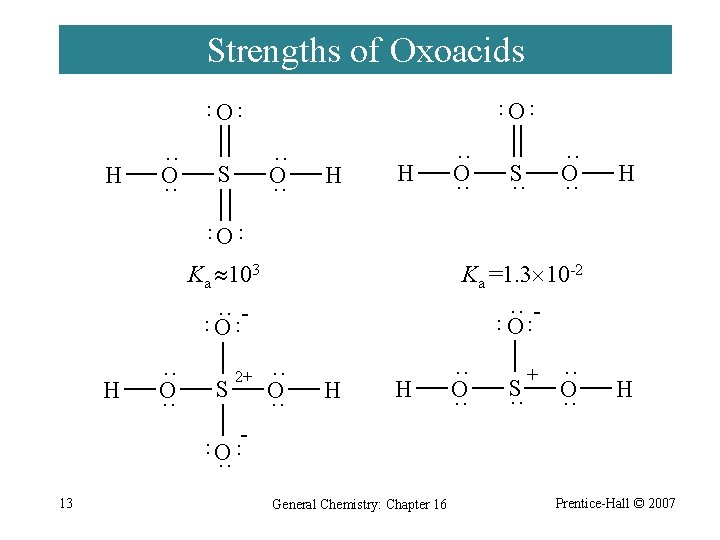
Strengths of Oxoacids ¨ Factors promoting electron withdrawal from the OH bond to the oxygen atom: · High electronegativity (EN) of the central atom. · A large number of terminal O atoms in the molecule. 12 H-O-Cl H-O-Br ENCl = 3. 0 ENBr= 2. 8 Ka = 2. 9 10 -8 Ka = 2. 1 10 -9 General Chemistry: Chapter 16 Prentice-Hall © 2007

Strengths of Oxoacids O ·· ·· O H ·· O ·· S H H ·· O ·· S ·· H ·· ·· O Ka 103 Ka =1. 3 10 -2 ·· O ·· ·· ·· O H ·· O ·· S ·· O ·· H H ·· O ·· S ·· + ·· O ·· H - ·· ·· O ·· 2+ 13 General Chemistry: Chapter 16 Prentice-Hall © 2007

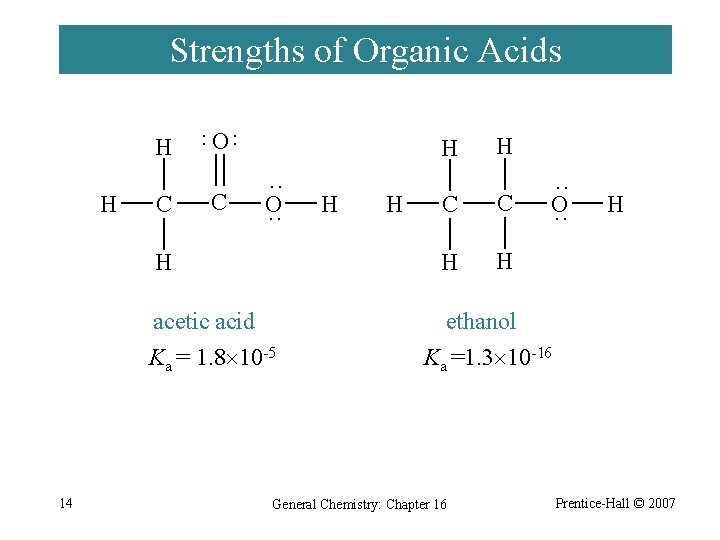
Strengths of Organic Acids H C O ·· ·· H C H ·· O ·· H acetic acid Ka = 1. 8 10 -5 14 H H H C C H H ·· O ·· H ethanol Ka =1. 3 10 -16 General Chemistry: Chapter 16 Prentice-Hall © 2007

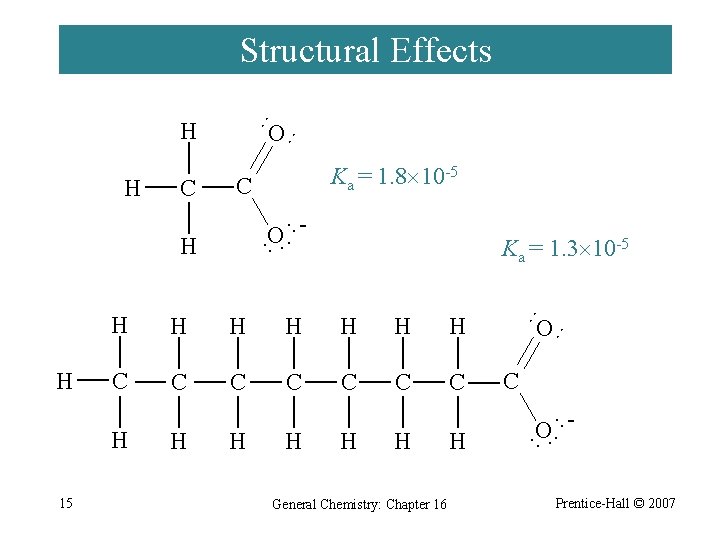
Structural Effects ·· C ·· O ·· H H H H C C C C H H General Chemistry: Chapter 16 H C O ·· ·· H O ·· H 15 Ka = 1. 3 10 -5 ·· H H Ka = 1. 8 10 -5 ·· C O ·· H Prentice-Hall © 2007