16 6 Integration The integration or area under



- Slides: 38

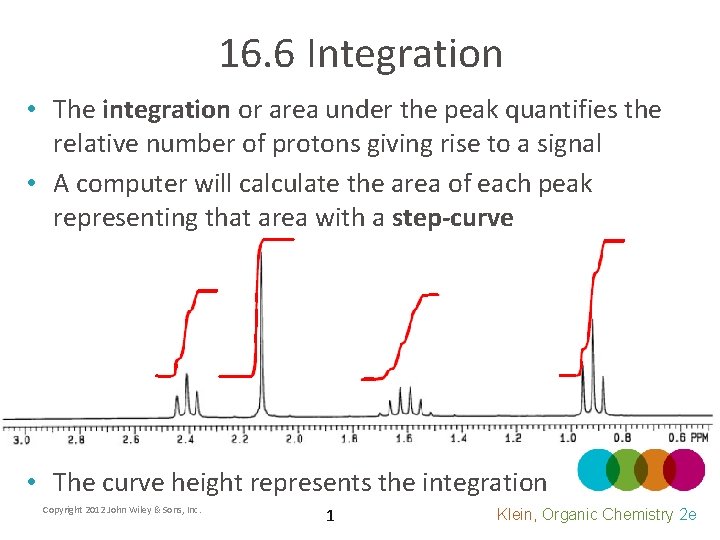
16. 6 Integration • The integration or area under the peak quantifies the relative number of protons giving rise to a signal • A computer will calculate the area of each peak representing that area with a step-curve • The curve height represents the integration Copyright 2012 John Wiley & Sons, Inc. 1 Klein, Organic Chemistry 2 e

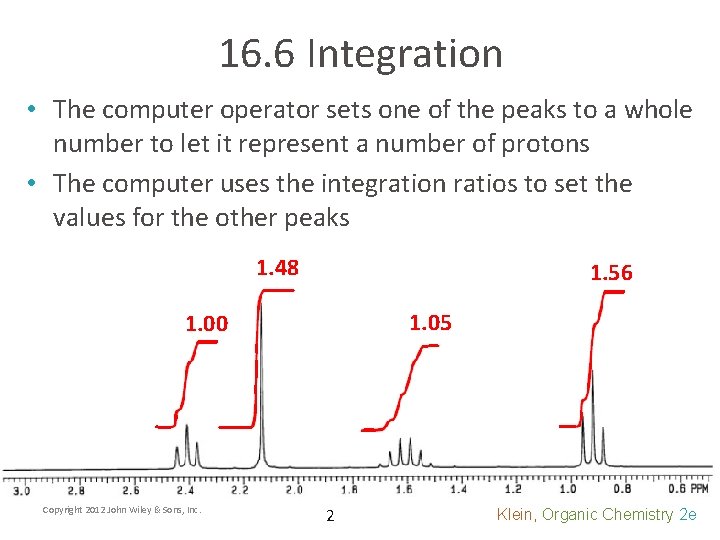
16. 6 Integration • The computer operator sets one of the peaks to a whole number to let it represent a number of protons • The computer uses the integration ratios to set the values for the other peaks 1. 48 1. 56 1. 05 1. 00 Copyright 2012 John Wiley & Sons, Inc. 2 Klein, Organic Chemistry 2 e

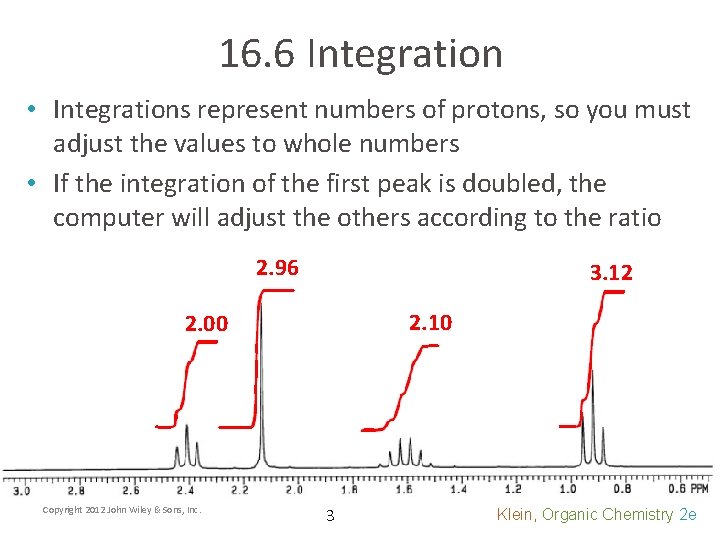
16. 6 Integration • Integrations represent numbers of protons, so you must adjust the values to whole numbers • If the integration of the first peak is doubled, the computer will adjust the others according to the ratio 2. 96 3. 12 2. 10 2. 00 Copyright 2012 John Wiley & Sons, Inc. 3 Klein, Organic Chemistry 2 e

16. 6 Integration • The integrations are relative quantities rather than an absolute count of the number of protons • Predict the 1 H shifts and integrations for tert-butyl methyl ether • Symmetry can also affect integrations • Predict the 1 H shifts and integrations for 3 -pentanone • Practice with Skill. Builder 16. 4 Copyright 2012 John Wiley & Sons, Inc. 4 Klein, Organic Chemistry 2 e

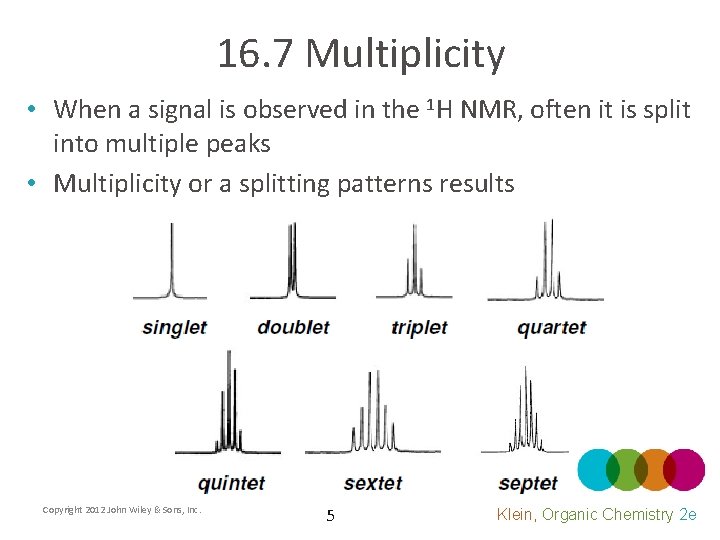
16. 7 Multiplicity • When a signal is observed in the 1 H NMR, often it is split into multiple peaks • Multiplicity or a splitting patterns results Copyright 2012 John Wiley & Sons, Inc. 5 Klein, Organic Chemistry 2 e

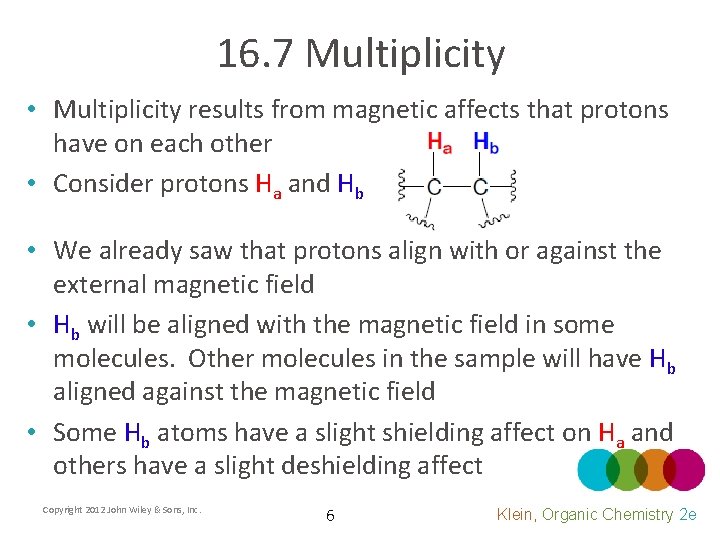
16. 7 Multiplicity • Multiplicity results from magnetic affects that protons have on each other • Consider protons Ha and Hb • We already saw that protons align with or against the external magnetic field • Hb will be aligned with the magnetic field in some molecules. Other molecules in the sample will have Hb aligned against the magnetic field • Some Hb atoms have a slight shielding affect on Ha and others have a slight deshielding affect Copyright 2012 John Wiley & Sons, Inc. 6 Klein, Organic Chemistry 2 e

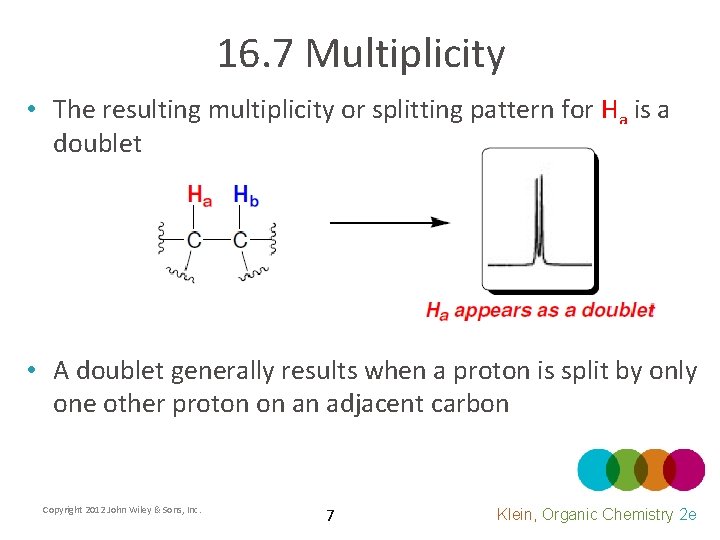
16. 7 Multiplicity • The resulting multiplicity or splitting pattern for Ha is a doublet • A doublet generally results when a proton is split by only one other proton on an adjacent carbon Copyright 2012 John Wiley & Sons, Inc. 7 Klein, Organic Chemistry 2 e

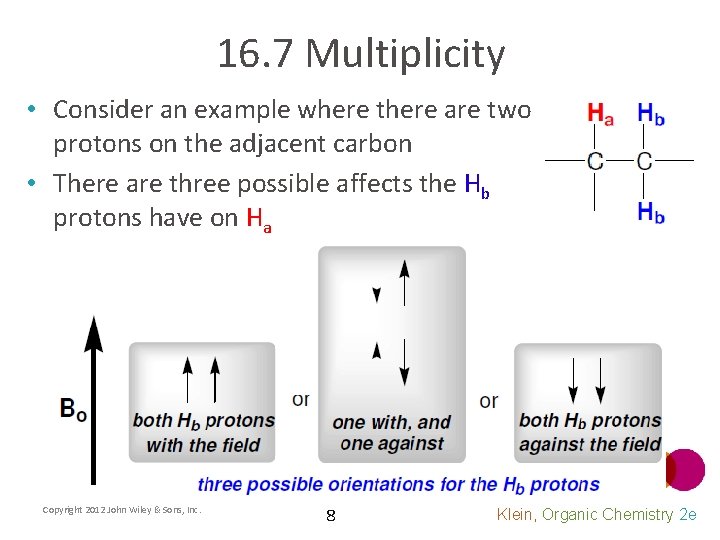
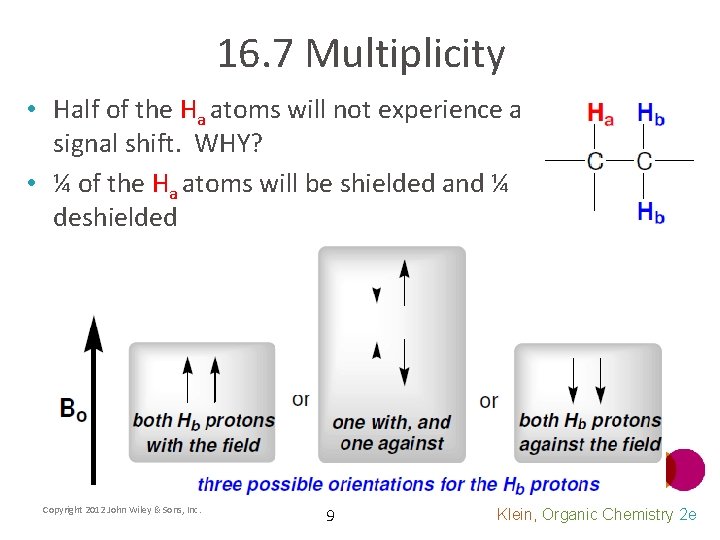
16. 7 Multiplicity • Consider an example where there are two protons on the adjacent carbon • There are three possible affects the Hb protons have on Ha Copyright 2012 John Wiley & Sons, Inc. 8 Klein, Organic Chemistry 2 e

16. 7 Multiplicity • Half of the Ha atoms will not experience a signal shift. WHY? • ¼ of the Ha atoms will be shielded and ¼ deshielded Copyright 2012 John Wiley & Sons, Inc. 9 Klein, Organic Chemistry 2 e

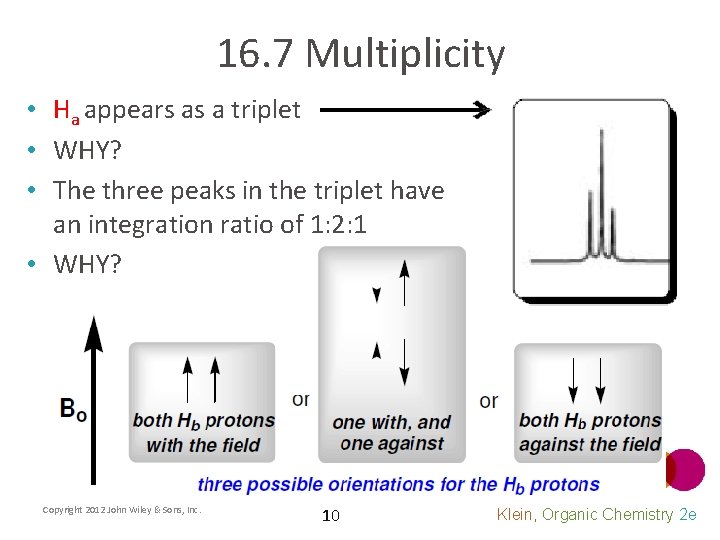
16. 7 Multiplicity • Ha appears as a triplet • WHY? • The three peaks in the triplet have an integration ratio of 1: 2: 1 • WHY? Copyright 2012 John Wiley & Sons, Inc. 10 Klein, Organic Chemistry 2 e

16. 7 Multiplicity • Consider a scenario where Ha has three equivalent Hb atoms splitting it • Explain how the magnetic fields cause shielding or deshielding Copyright 2012 John Wiley & Sons, Inc. 11 Klein, Organic Chemistry 2 e

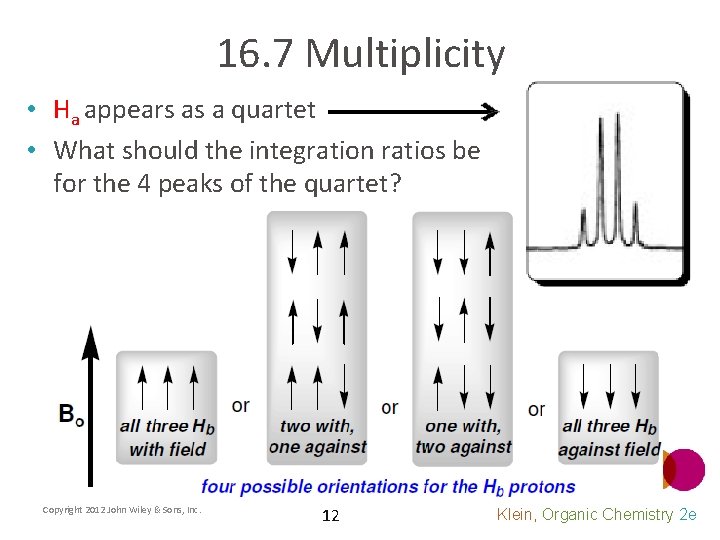
16. 7 Multiplicity • Ha appears as a quartet • What should the integration ratios be for the 4 peaks of the quartet? Copyright 2012 John Wiley & Sons, Inc. 12 Klein, Organic Chemistry 2 e

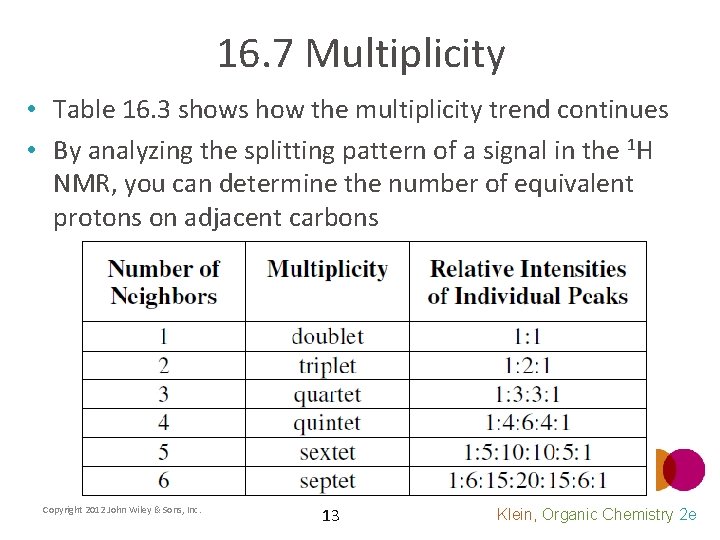
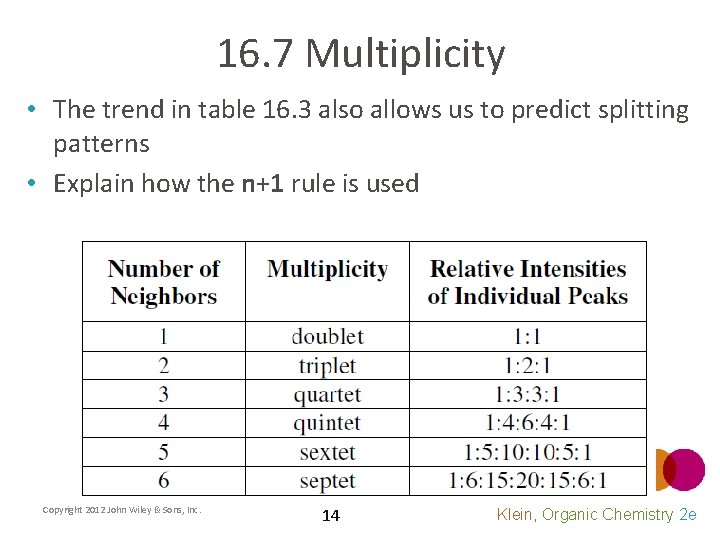
16. 7 Multiplicity • Table 16. 3 shows how the multiplicity trend continues • By analyzing the splitting pattern of a signal in the 1 H NMR, you can determine the number of equivalent protons on adjacent carbons Copyright 2012 John Wiley & Sons, Inc. 13 Klein, Organic Chemistry 2 e

16. 7 Multiplicity • The trend in table 16. 3 also allows us to predict splitting patterns • Explain how the n+1 rule is used Copyright 2012 John Wiley & Sons, Inc. 14 Klein, Organic Chemistry 2 e

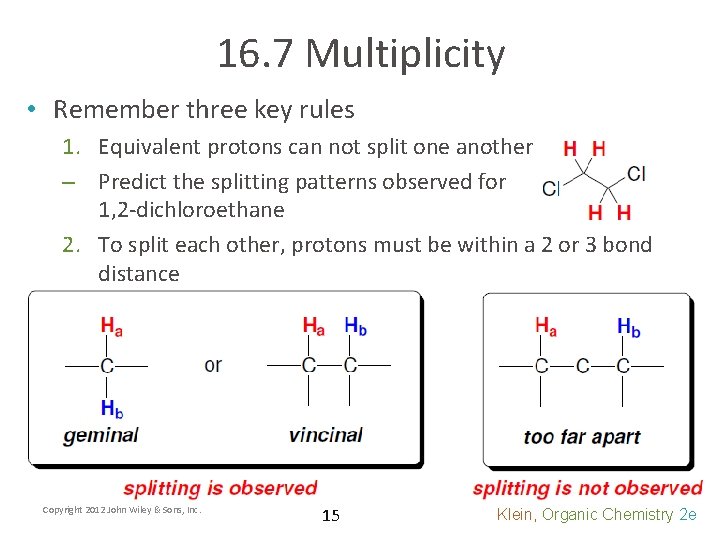
16. 7 Multiplicity • Remember three key rules 1. Equivalent protons can not split one another – Predict the splitting patterns observed for 1, 2 -dichloroethane 2. To split each other, protons must be within a 2 or 3 bond distance Copyright 2012 John Wiley & Sons, Inc. 15 Klein, Organic Chemistry 2 e

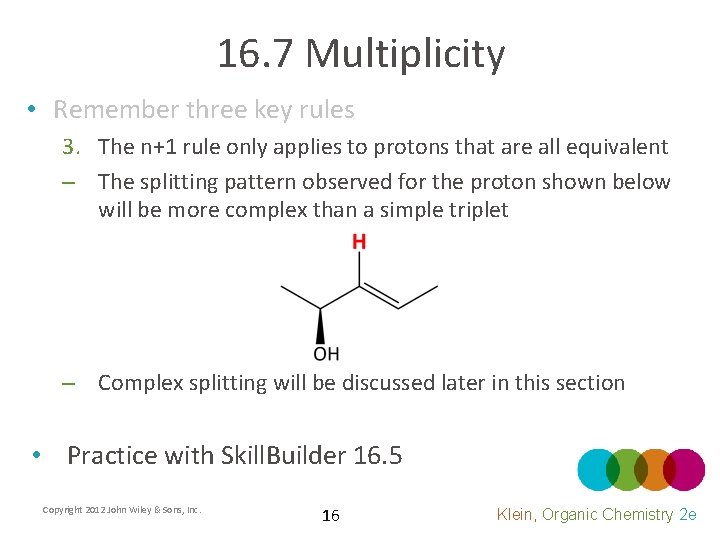
16. 7 Multiplicity • Remember three key rules 3. The n+1 rule only applies to protons that are all equivalent – The splitting pattern observed for the proton shown below will be more complex than a simple triplet – Complex splitting will be discussed later in this section • Practice with Skill. Builder 16. 5 Copyright 2012 John Wiley & Sons, Inc. 16 Klein, Organic Chemistry 2 e

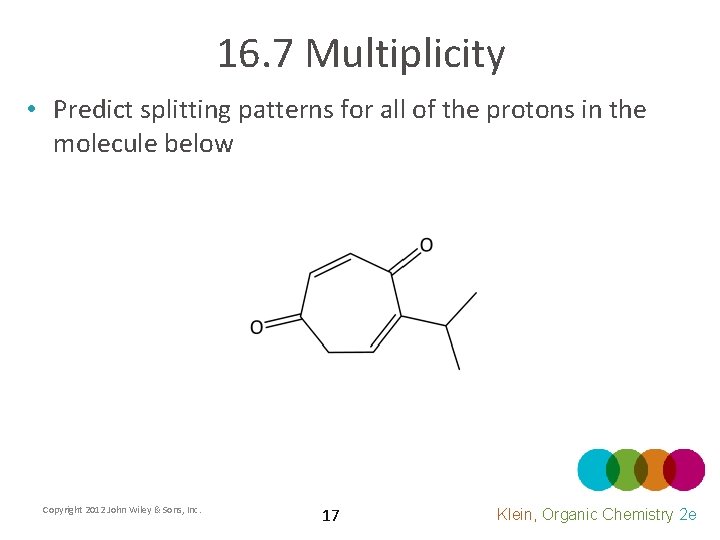
16. 7 Multiplicity • Predict splitting patterns for all of the protons in the molecule below Copyright 2012 John Wiley & Sons, Inc. 17 Klein, Organic Chemistry 2 e

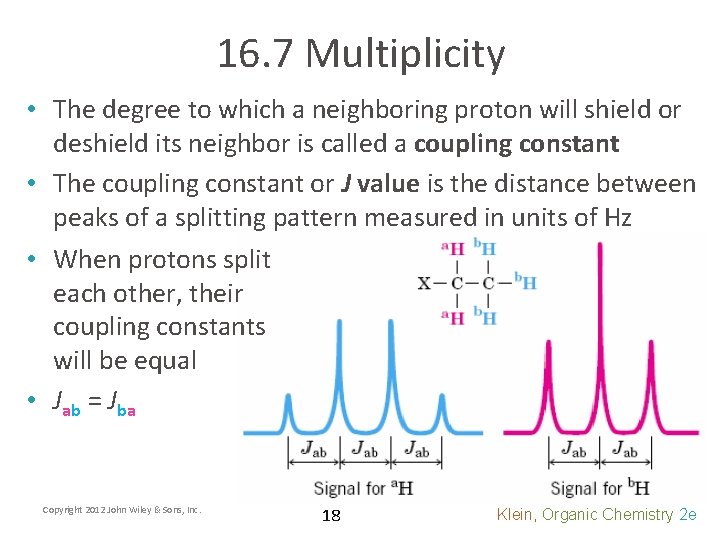
16. 7 Multiplicity • The degree to which a neighboring proton will shield or deshield its neighbor is called a coupling constant • The coupling constant or J value is the distance between peaks of a splitting pattern measured in units of Hz • When protons split each other, their coupling constants will be equal • Jab = Jba Copyright 2012 John Wiley & Sons, Inc. 18 Klein, Organic Chemistry 2 e

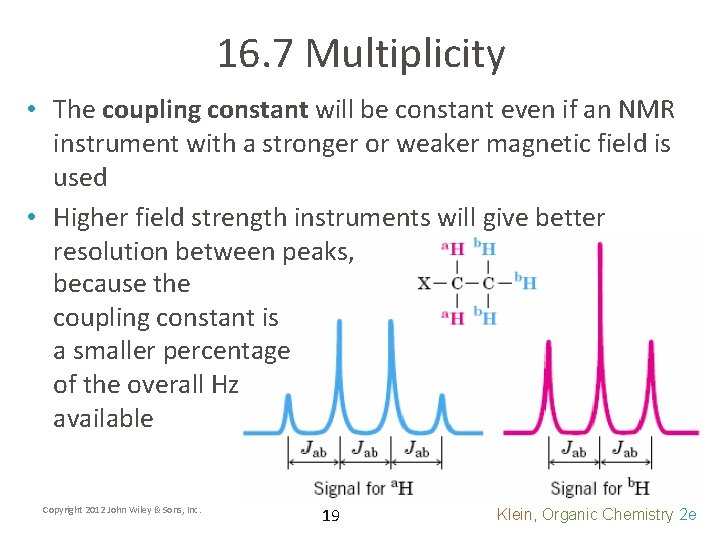
16. 7 Multiplicity • The coupling constant will be constant even if an NMR instrument with a stronger or weaker magnetic field is used • Higher field strength instruments will give better resolution between peaks, because the coupling constant is a smaller percentage of the overall Hz available Copyright 2012 John Wiley & Sons, Inc. 19 Klein, Organic Chemistry 2 e

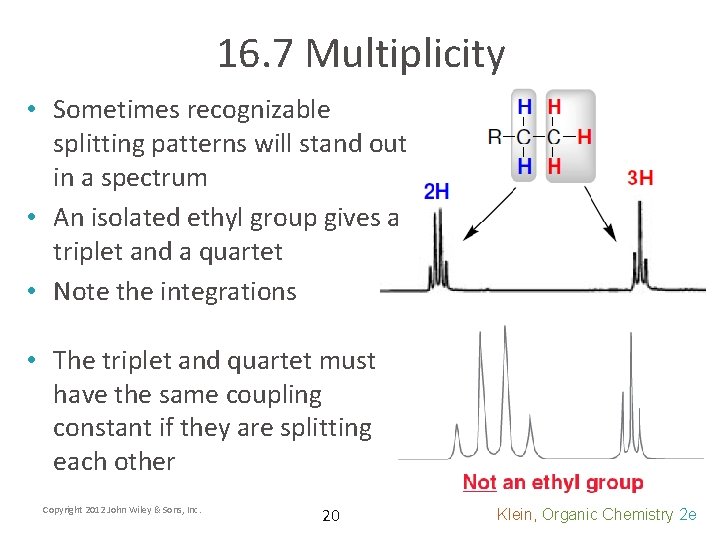
16. 7 Multiplicity • Sometimes recognizable splitting patterns will stand out in a spectrum • An isolated ethyl group gives a triplet and a quartet • Note the integrations • The triplet and quartet must have the same coupling constant if they are splitting each other Copyright 2012 John Wiley & Sons, Inc. 20 Klein, Organic Chemistry 2 e

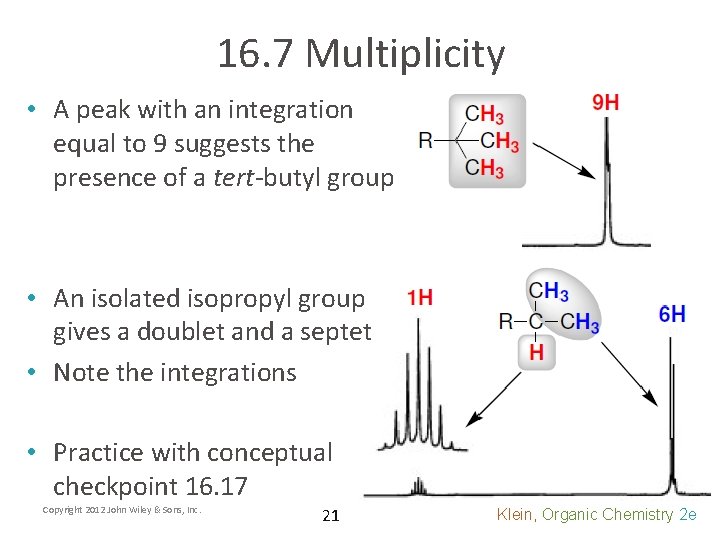
16. 7 Multiplicity • A peak with an integration equal to 9 suggests the presence of a tert-butyl group • An isolated isopropyl group gives a doublet and a septet • Note the integrations • Practice with conceptual checkpoint 16. 17 Copyright 2012 John Wiley & Sons, Inc. 21 Klein, Organic Chemistry 2 e

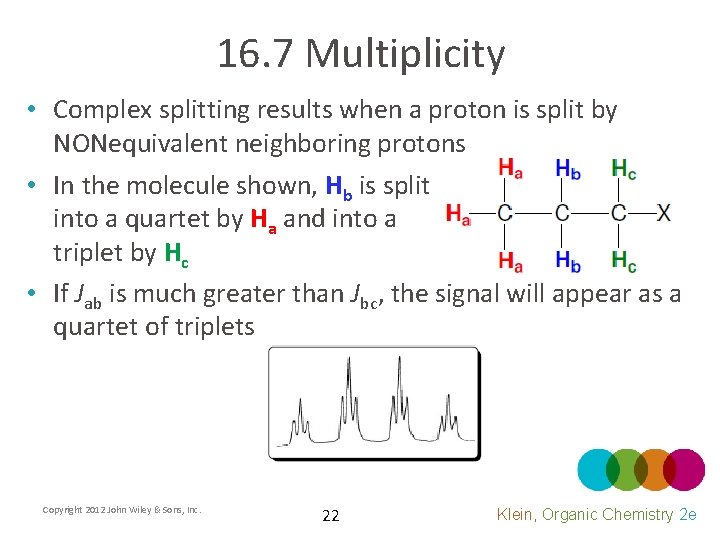
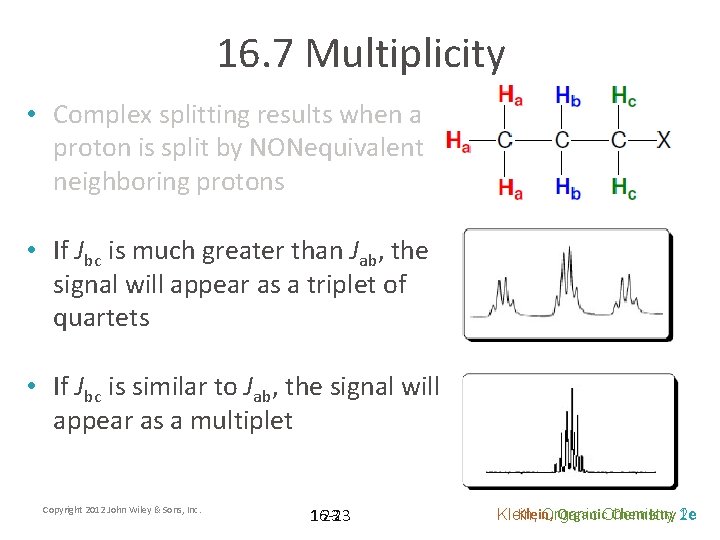
16. 7 Multiplicity • Complex splitting results when a proton is split by NONequivalent neighboring protons • In the molecule shown, Hb is split into a quartet by Ha and into a triplet by Hc • If Jab is much greater than Jbc, the signal will appear as a quartet of triplets Copyright 2012 John Wiley & Sons, Inc. 22 Klein, Organic Chemistry 2 e

16. 7 Multiplicity • Complex splitting results when a proton is split by NONequivalent neighboring protons • If Jbc is much greater than Jab, the signal will appear as a triplet of quartets • If Jbc is similar to Jab, the signal will appear as a multiplet Copyright 2012 John Wiley & Sons, Inc. 16 -23 23 Klein, Organic. Chemistry 2 e 1 e Klein, Organic

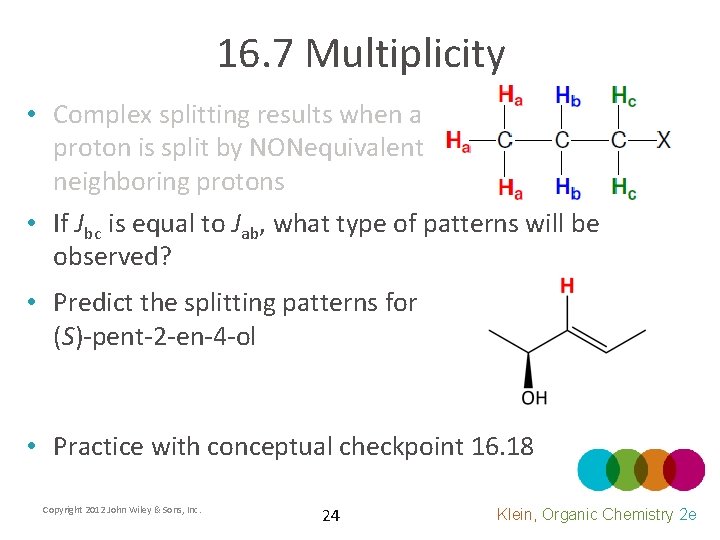
16. 7 Multiplicity • Complex splitting results when a proton is split by NONequivalent neighboring protons • If Jbc is equal to Jab, what type of patterns will be observed? • Predict the splitting patterns for (S)-pent-2 -en-4 -ol • Practice with conceptual checkpoint 16. 18 Copyright 2012 John Wiley & Sons, Inc. 24 Klein, Organic Chemistry 2 e

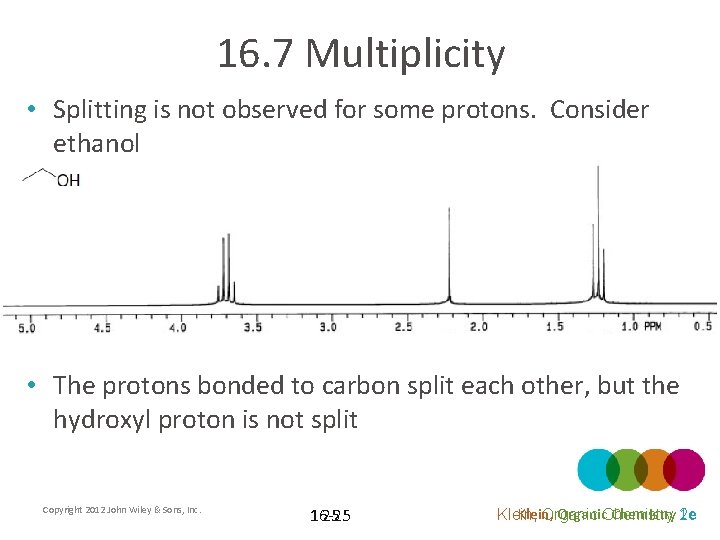
16. 7 Multiplicity • Splitting is not observed for some protons. Consider ethanol • The protons bonded to carbon split each other, but the hydroxyl proton is not split Copyright 2012 John Wiley & Sons, Inc. 16 -25 25 Klein, Organic. Chemistry 2 e 1 e Klein, Organic

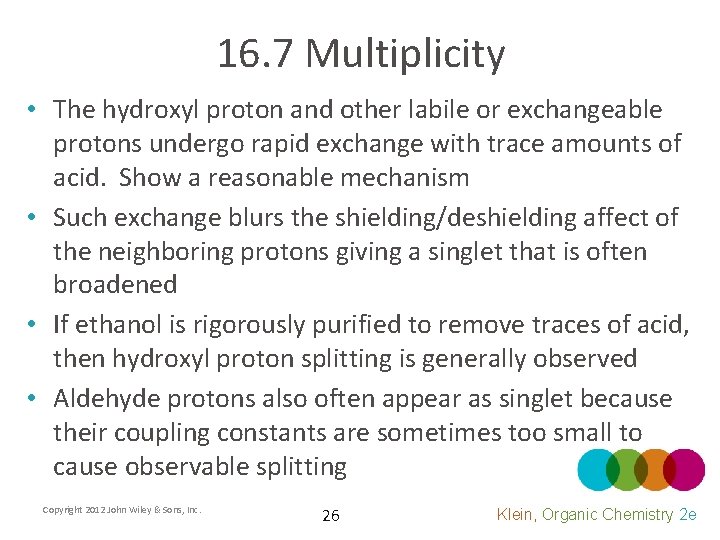
16. 7 Multiplicity • The hydroxyl proton and other labile or exchangeable protons undergo rapid exchange with trace amounts of acid. Show a reasonable mechanism • Such exchange blurs the shielding/deshielding affect of the neighboring protons giving a singlet that is often broadened • If ethanol is rigorously purified to remove traces of acid, then hydroxyl proton splitting is generally observed • Aldehyde protons also often appear as singlet because their coupling constants are sometimes too small to cause observable splitting Copyright 2012 John Wiley & Sons, Inc. 26 Klein, Organic Chemistry 2 e

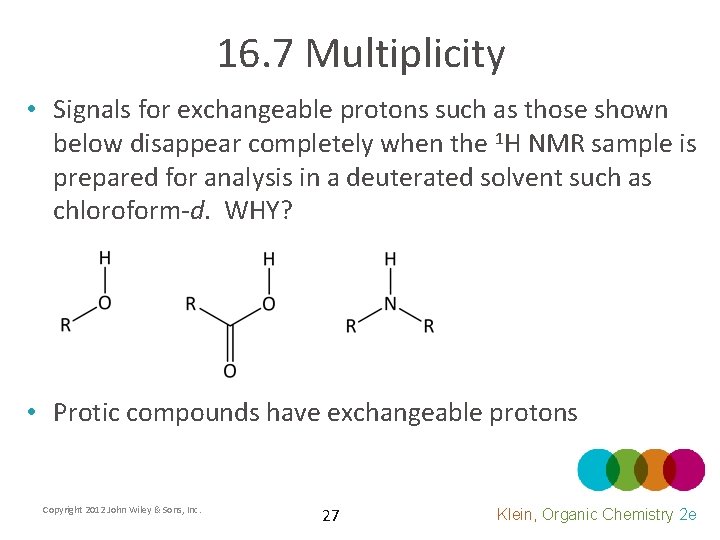
16. 7 Multiplicity • Signals for exchangeable protons such as those shown below disappear completely when the 1 H NMR sample is prepared for analysis in a deuterated solvent such as chloroform-d. WHY? • Protic compounds have exchangeable protons Copyright 2012 John Wiley & Sons, Inc. 27 Klein, Organic Chemistry 2 e

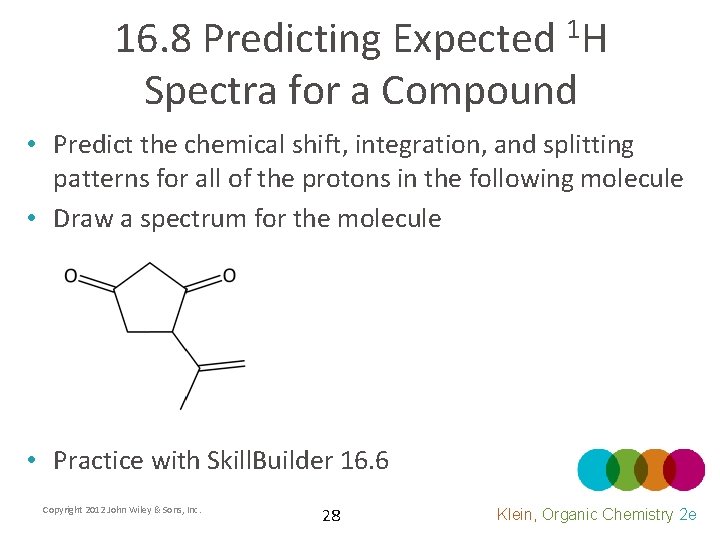
16. 8 Predicting Expected 1 H Spectra for a Compound • Predict the chemical shift, integration, and splitting patterns for all of the protons in the following molecule • Draw a spectrum for the molecule • Practice with Skill. Builder 16. 6 Copyright 2012 John Wiley & Sons, Inc. 28 Klein, Organic Chemistry 2 e

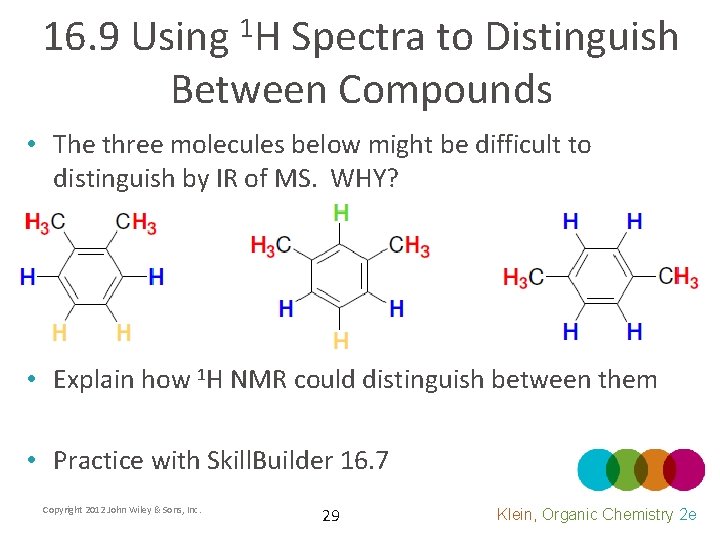
16. 9 Using 1 H Spectra to Distinguish Between Compounds • The three molecules below might be difficult to distinguish by IR of MS. WHY? • Explain how 1 H NMR could distinguish between them • Practice with Skill. Builder 16. 7 Copyright 2012 John Wiley & Sons, Inc. 29 Klein, Organic Chemistry 2 e

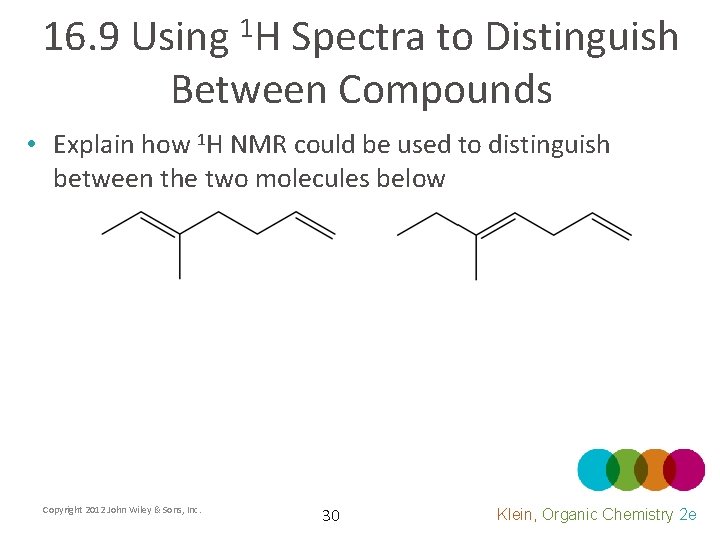
16. 9 Using 1 H Spectra to Distinguish Between Compounds • Explain how 1 H NMR could be used to distinguish between the two molecules below Copyright 2012 John Wiley & Sons, Inc. 30 Klein, Organic Chemistry 2 e

16. 10 Analyzing a 1 H NMR Spectrum • With a given formula and 1 H NMR spectrum, you can determine a molecule’s structure by a 4 -step process 1. Calculate the degree or unsaturation or hydrogen deficiency index (HDI). What does the HDI tell you? 2. Consider the number of NMR signals and integration to look for symmetry in the molecule 3. Analyze each signal, and draw molecular fragments that match the shift, integration, and multiplicity 4. Assemble the fragments into a complete structure like puzzle pieces • Practice with Skill. Builder 16. 8 Copyright 2012 John Wiley & Sons, Inc. 31 Klein, Organic Chemistry 2 e

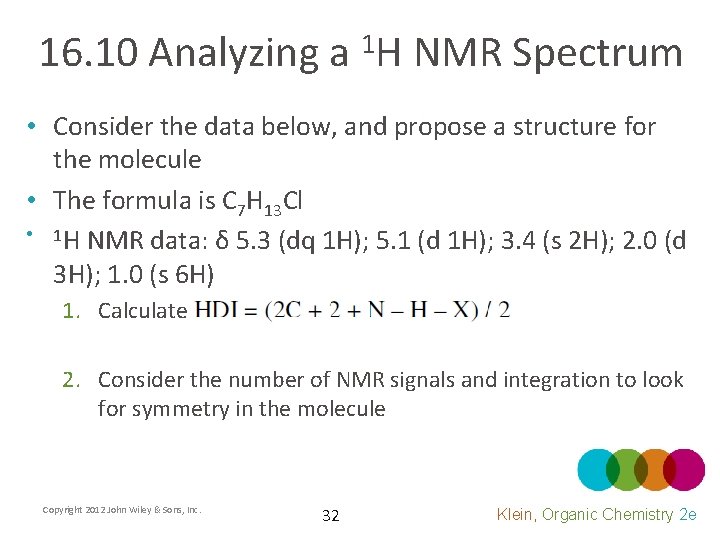
16. 10 Analyzing a 1 H NMR Spectrum • Consider the data below, and propose a structure for the molecule • The formula is C 7 H 13 Cl • 1 H NMR data: δ 5. 3 (dq 1 H); 5. 1 (d 1 H); 3. 4 (s 2 H); 2. 0 (d 3 H); 1. 0 (s 6 H) 1. Calculate 2. Consider the number of NMR signals and integration to look for symmetry in the molecule Copyright 2012 John Wiley & Sons, Inc. 32 Klein, Organic Chemistry 2 e

16. 10 Analyzing a 1 H NMR Spectrum • Consider the data below, and propose a structure for the molecule • The formula is C 7 H 13 Cl • 1 H NMR data: δ 5. 3 (dq 1 H); 5. 1 (d 1 H); 3. 4 (s 2 H); 2. 0 (d 3 H); 1. 0 (s 6 H) 3. Analyze each signal, and draw molecular fragments that match the shift, integration, and multiplicity 4. Assemble the fragments into a complete structure like puzzle pieces Copyright 2012 John Wiley & Sons, Inc. 33 Klein, Organic Chemistry 2 e

16. 11 Acquiring a 13 C NMR Spectrum • Because 1 H is by far the most abundant isotope of hydrogen, 1 H NMR signals are generally strong • 13 C only accounts for about 1% of carbon atoms in nature, so a sensitive receiver coil and/or concentrated NMR sample is needed • In 1 H NMR, shift, splitting, and integration are important • In 13 C NMR, only the number of signals and the shift will be considered Copyright 2012 John Wiley & Sons, Inc. 34 Klein, Organic Chemistry 2 e

16. 11 Acquiring a 13 C NMR Spectrum • In 13 C NMR, the 1 H-13 C splitting is often so complex that the spectrum is unreadable • To elucidate the 13 C spectrum and make it easier to determine the total number of 13 C signals, 13 C NMR are generally decoupled • In the vast majority of 13 C spectra, all of the signals are singlets Copyright 2012 John Wiley & Sons, Inc. 35 Klein, Organic Chemistry 2 e

Study Guide for sections 16. 6 -16. 11 DAY 23, Terms to know: Sections 16. 6 -16. 11 integration, splitting pattern or multiplicity, homotopic, enantiotopic, diastereotopic, internal standard, downfield, upfield, diamagnetic anisotropy, aromatic, coupling constant, exchangeable proton, decoupled DAY 23, Specific outcomes and skills that may be tested on exam 4: Sections 16. 6 -16. 11 • Be able to explain what integration values represent in an NMR. • Given a molecular structure, predict the integrations of each signal expected. • Be able to label symmetrical splitting patterns such as singlet, double, triplet, etc. • Be able to predict splitting patterns for each signal in a proton NMR based on the n+1 rule. • Be able to identify H atoms that may have splitting patterns more complex than can be determined using the n+1 rule. • Be able to explain how nonequivalent H atoms on adjacent carbons can split an atom with the same coupling constant giving a signal that would be predicted from the n+1 rule or with different coupling constants giving a more complex signal. • Be able to identify some complex splitting patterns such as doublet of doublets, triplet of quartets, etc. • Given a molecular structure, be able to predict some complex splitting patterns. • Be able to explain how the NMR spectra is affected when a proton is exchangeable. • Given a series of proton NMR peak data, be able to propose a reasonable molecular structure for the molecule. 36 Klein, Organic. Chemistry 2 e 2 e Klein, Organic

Practice Problems for sections 16. 6 -16. 11 Complete these problems outside of class until you are confident you have learned the SKILLS in this section outlined on the study guide and we will review some of them next class period. 16. 11 16. 12 16. 13 16. 15 16. 17 16. 19 16. 21 16. 22 16. 23 16. 37 16. 40 16. 41 16. 44 16. 45 16. 48 37 Klein, Organic. Chemistry 2 e 2 e Klein, Organic

Prep for Day 24 Must Watch videos: https: //www. youtube. com/watch? v=HBXVq_Ju. BI 8 (13 C NMR) https: //www. youtube. com/watch? v=qu 2 de. F 8 cjwk (addition reactions intro) https: //www. youtube. com/watch? v=g. YOIQ 0 z. Tig. Q (hydrohalogenation) https: //www. youtube. com/watch? v=FOms 5 Hf. Ju. Pw (hydration) Other helpful videos: https: //www. youtube. com/watch? v=8 h. L 3 GXCttuo (13 C NMR example) https: //www. youtube. com/watch? v=Brv. NZt. IKSr 4 (13 C DEPT NMR) https: //www. youtube. com/watch? v=i. EKA 0 j. Ust. Ps (hydrohalogenation) https: //www. youtube. com/watch? v=s. Ei. Qm 7 Iz. Ug 8 (hydration) http: //ps. uci. edu/content/chem-51 b-organic-chemistry (lecture 6 -7) Read sections 16. 12 -16. 13, 9. 1 -9. 4 38 Klein, Organic. Chemistry 2 e 2 e Klein, Organic