16 34 The following pictures represent solutions that
- Slides: 11




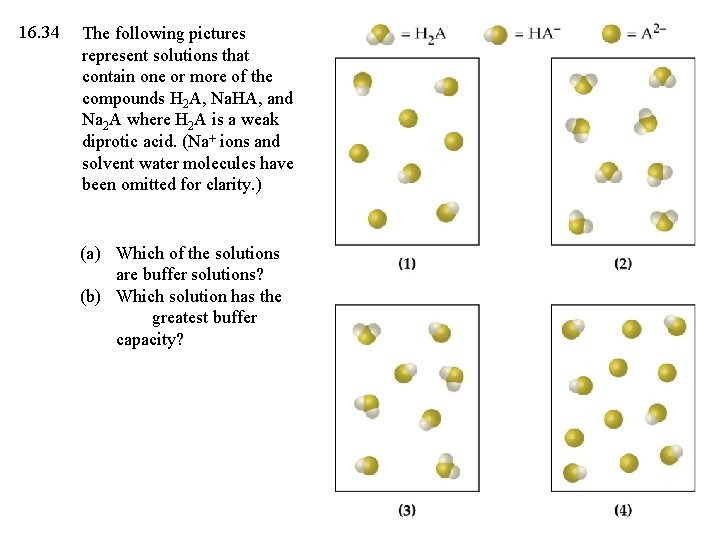
16. 34 The following pictures represent solutions that contain one or more of the compounds H 2 A, Na. HA, and Na 2 A where H 2 A is a weak diprotic acid. (Na+ ions and solvent water molecules have been omitted for clarity. ) (a) Which of the solutions are buffer solutions? (b) Which solution has the greatest buffer capacity?

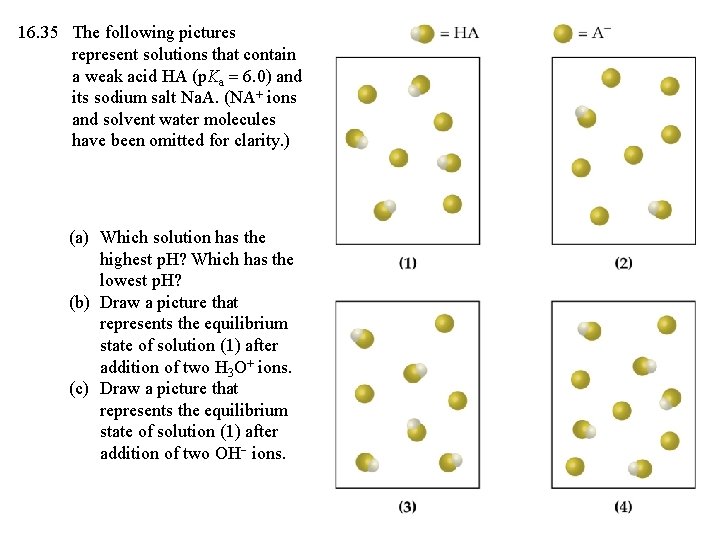
16. 35 The following pictures represent solutions that contain a weak acid HA (p. Ka = 6. 0) and its sodium salt Na. A. (NA+ ions and solvent water molecules have been omitted for clarity. ) (a) Which solution has the highest p. H? Which has the lowest p. H? (b) Draw a picture that represents the equilibrium state of solution (1) after addition of two H 3 O+ ions. (c) Draw a picture that represents the equilibrium state of solution (1) after addition of two OH ions.

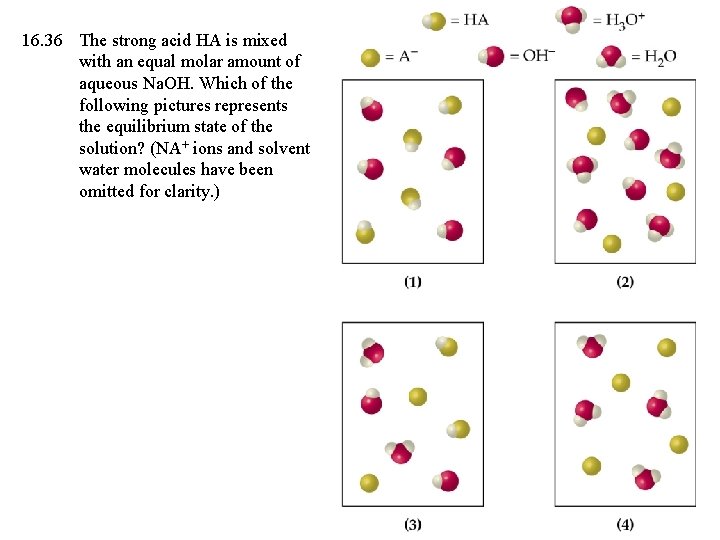
16. 36 The strong acid HA is mixed with an equal molar amount of aqueous Na. OH. Which of the following pictures represents the equilibrium state of the solution? (NA+ ions and solvent water molecules have been omitted for clarity. )

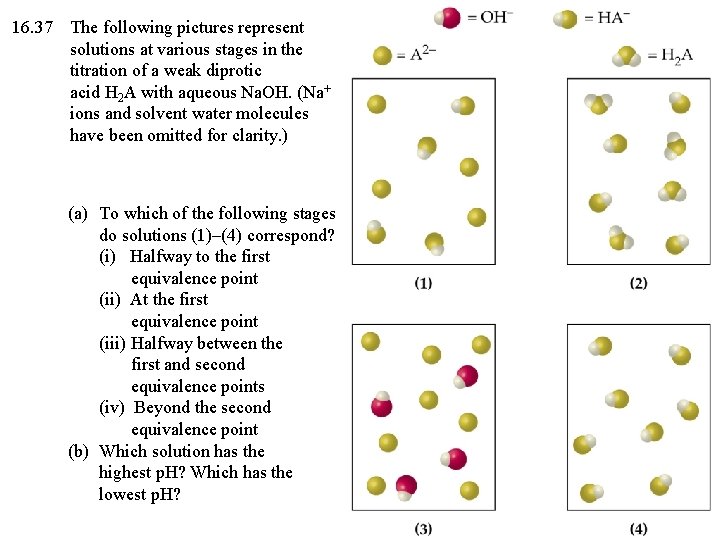
16. 37 The following pictures represent solutions at various stages in the titration of a weak diprotic acid H 2 A with aqueous Na. OH. (Na+ ions and solvent water molecules have been omitted for clarity. ) (a) To which of the following stages do solutions (1)–(4) correspond? (i) Halfway to the first equivalence point (ii) At the first equivalence point (iii) Halfway between the first and second equivalence points (iv) Beyond the second equivalence point (b) Which solution has the highest p. H? Which has the lowest p. H?

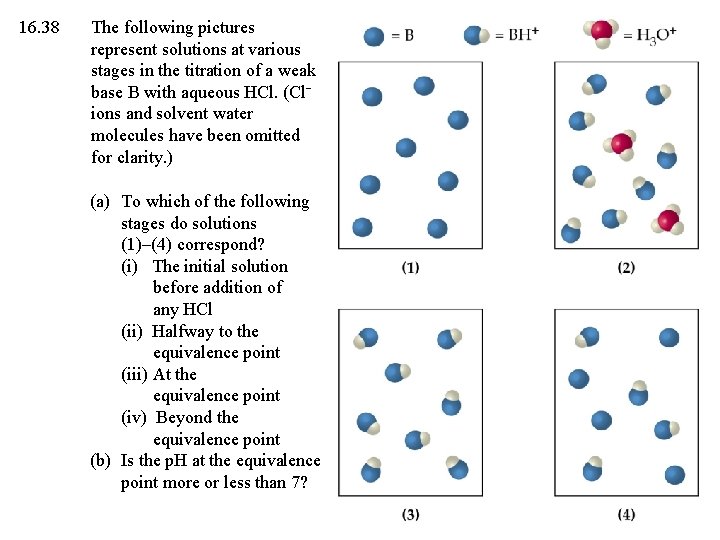
16. 38 The following pictures represent solutions at various stages in the titration of a weak base B with aqueous HCl. (Cl ions and solvent water molecules have been omitted for clarity. ) (a) To which of the following stages do solutions (1)–(4) correspond? (i) The initial solution before addition of any HCl (ii) Halfway to the equivalence point (iii) At the equivalence point (iv) Beyond the equivalence point (b) Is the p. H at the equivalence point more or less than 7?

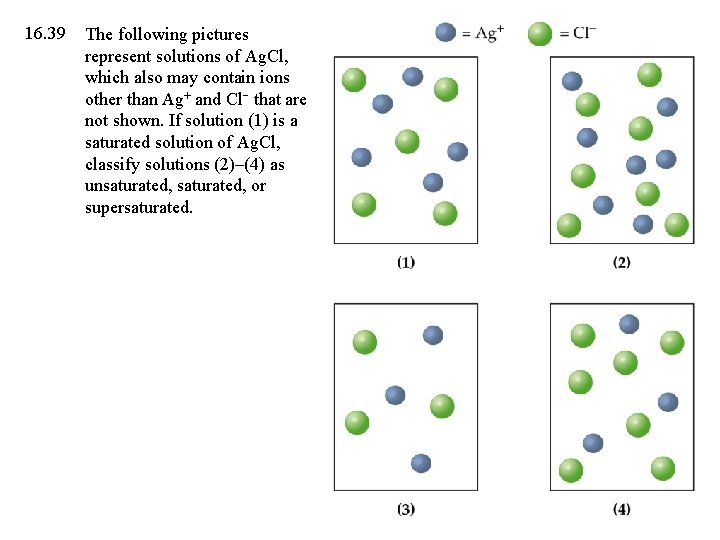
16. 39 The following pictures represent solutions of Ag. Cl, which also may contain ions other than Ag+ and Cl that are not shown. If solution (1) is a saturated solution of Ag. Cl, classify solutions (2)–(4) as unsaturated, or supersaturated.

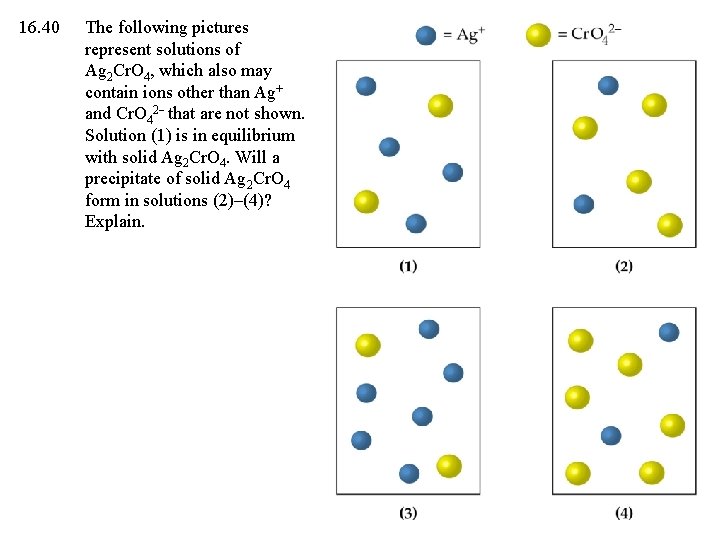
16. 40 The following pictures represent solutions of Ag 2 Cr. O 4, which also may contain ions other than Ag+ and Cr. O 42 that are not shown. Solution (1) is in equilibrium with solid Ag 2 Cr. O 4. Will a precipitate of solid Ag 2 Cr. O 4 form in solutions (2)–(4)? Explain.

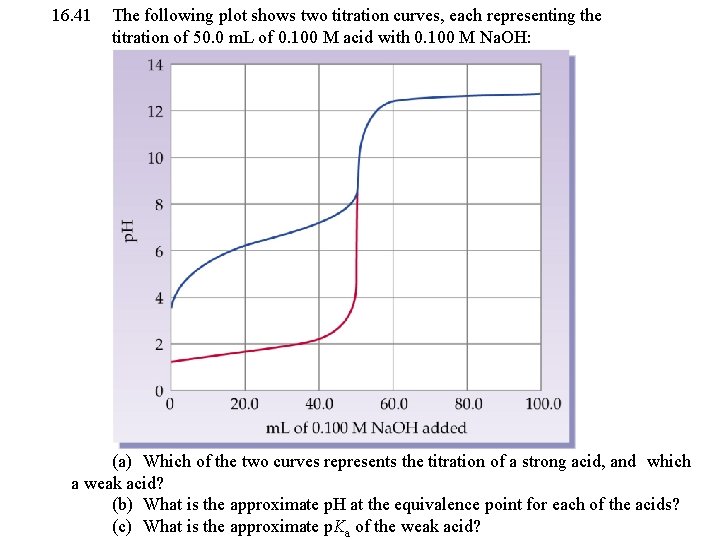
16. 41 The following plot shows two titration curves, each representing the titration of 50. 0 m. L of 0. 100 M acid with 0. 100 M Na. OH: (a) Which of the two curves represents the titration of a strong acid, and which a weak acid? (b) What is the approximate p. H at the equivalence point for each of the acids? (c) What is the approximate p. Ka of the weak acid?