16 1 Intro to NMR Spectroscopy What is



- Slides: 41

16. 1 Intro to NMR Spectroscopy • What is spectroscopy? • Nuclear Magnetic Resonance (NMR) spectroscopy may be the most powerful method of gaining structural information about organic compounds • NMR involves an interaction between electromagnetic radiation (light) and the nucleus of an atom – We will focus on C and H nuclei. WHY? – The structure (connectivity) of a molecule affects how the radiation interacts with each nucleus in the molecule Copyright 2012 John Wiley & Sons, Inc. 1 Klein, Organic Chemistry 2 e

16. 1 Intro to NMR Spectroscopy • Protons and neutrons in a nucleus behave as if they are spinning • If the total number of neutrons and protons is an ODD number, the atoms will have net nuclear spin • Examples: • The spinning charge in the nucleus creates a magnetic moment • We saw in Chapter 15 how a dipole moment creates an electric field • What does a magnetic moment create? Copyright 2012 John Wiley & Sons, Inc. 2 Klein, Organic Chemistry 2 e

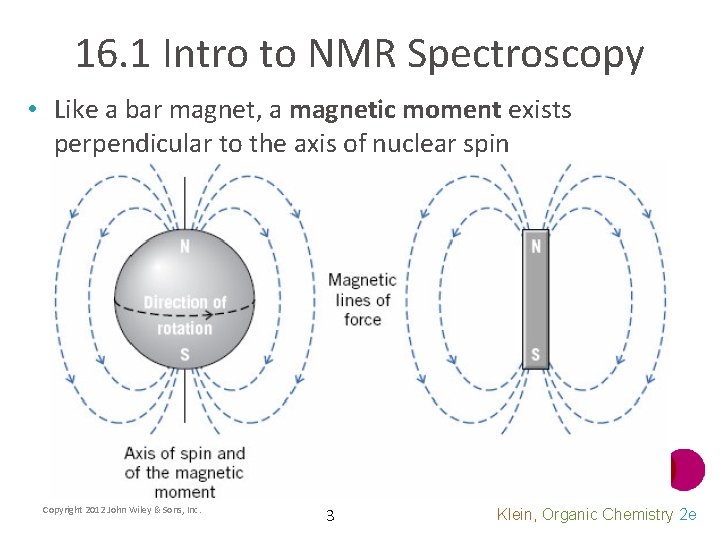
16. 1 Intro to NMR Spectroscopy • Like a bar magnet, a magnetic moment exists perpendicular to the axis of nuclear spin Copyright 2012 John Wiley & Sons, Inc. 3 Klein, Organic Chemistry 2 e

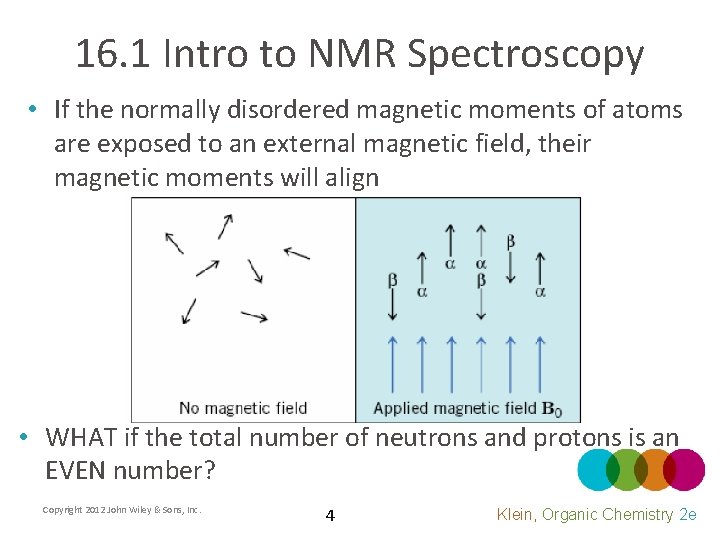
16. 1 Intro to NMR Spectroscopy • If the normally disordered magnetic moments of atoms are exposed to an external magnetic field, their magnetic moments will align • WHAT if the total number of neutrons and protons is an EVEN number? Copyright 2012 John Wiley & Sons, Inc. 4 Klein, Organic Chemistry 2 e

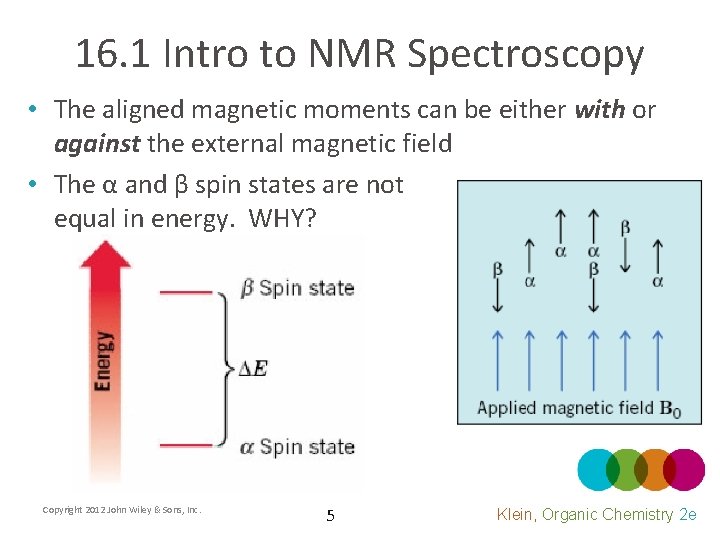
16. 1 Intro to NMR Spectroscopy • The aligned magnetic moments can be either with or against the external magnetic field • The α and β spin states are not equal in energy. WHY? Copyright 2012 John Wiley & Sons, Inc. 5 Klein, Organic Chemistry 2 e

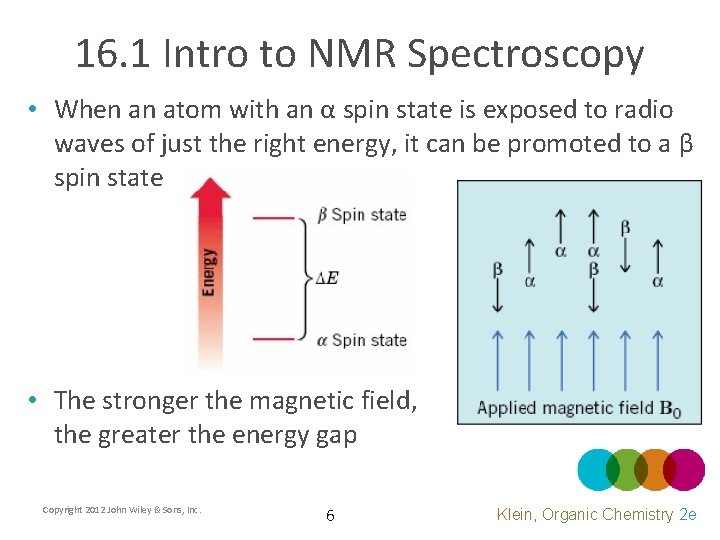
16. 1 Intro to NMR Spectroscopy • When an atom with an α spin state is exposed to radio waves of just the right energy, it can be promoted to a β spin state • The stronger the magnetic field, the greater the energy gap Copyright 2012 John Wiley & Sons, Inc. 6 Klein, Organic Chemistry 2 e

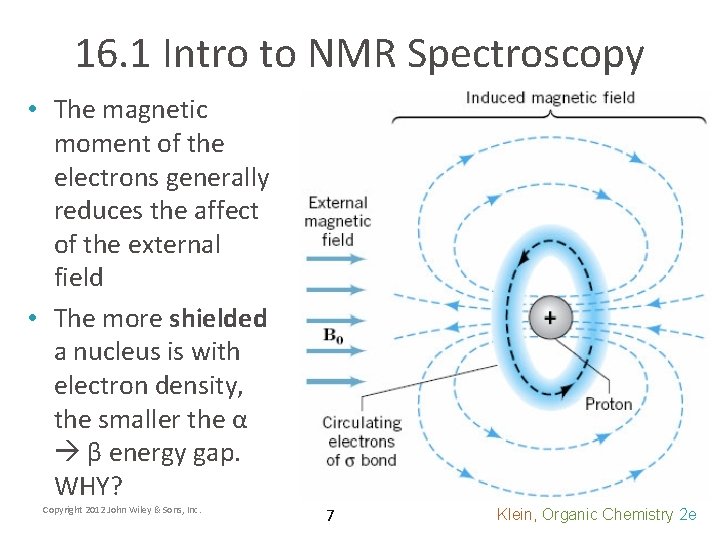
16. 1 Intro to NMR Spectroscopy • The magnetic moment of the electrons generally reduces the affect of the external field • The more shielded a nucleus is with electron density, the smaller the α β energy gap. WHY? Copyright 2012 John Wiley & Sons, Inc. 7 Klein, Organic Chemistry 2 e

16. 1 Intro to NMR Spectroscopy • The amount of radio wave energy necessary for the α β energy transition depends on the electronic environment for the atom • When the α spins are flipped to β spins, the atoms are said to be in resonance • The use of the term, “resonance” here is totally different from when we are talking about electrons in molecular orbitals • How does NMR spectroscopy tell us about molecular structure? Copyright 2012 John Wiley & Sons, Inc. 8 Klein, Organic Chemistry 2 e

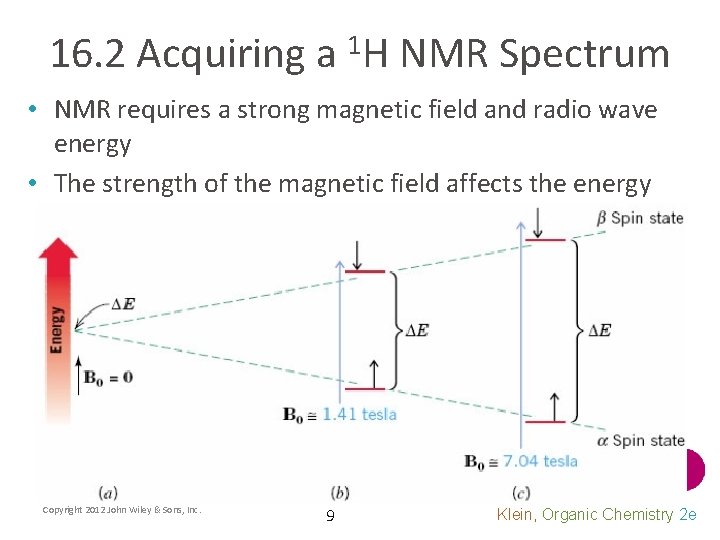
16. 2 Acquiring a 1 H NMR Spectrum • NMR requires a strong magnetic field and radio wave energy • The strength of the magnetic field affects the energy gap Copyright 2012 John Wiley & Sons, Inc. 9 Klein, Organic Chemistry 2 e

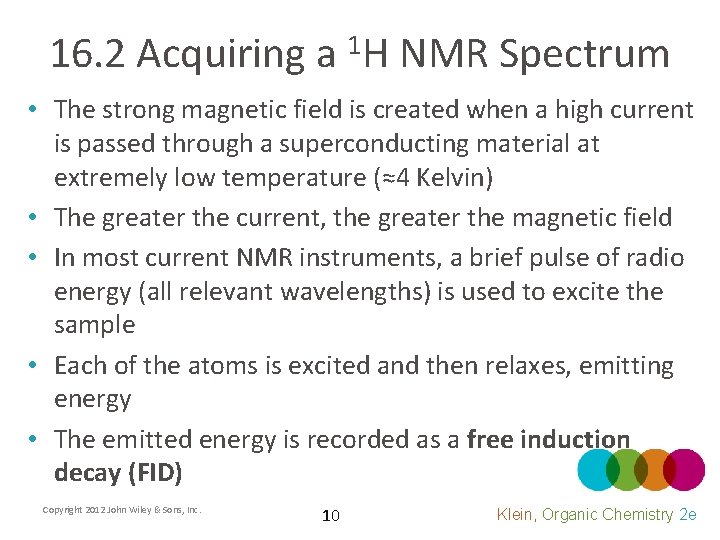
16. 2 Acquiring a 1 H NMR Spectrum • The strong magnetic field is created when a high current is passed through a superconducting material at extremely low temperature (≈4 Kelvin) • The greater the current, the greater the magnetic field • In most current NMR instruments, a brief pulse of radio energy (all relevant wavelengths) is used to excite the sample • Each of the atoms is excited and then relaxes, emitting energy • The emitted energy is recorded as a free induction decay (FID) Copyright 2012 John Wiley & Sons, Inc. 10 Klein, Organic Chemistry 2 e

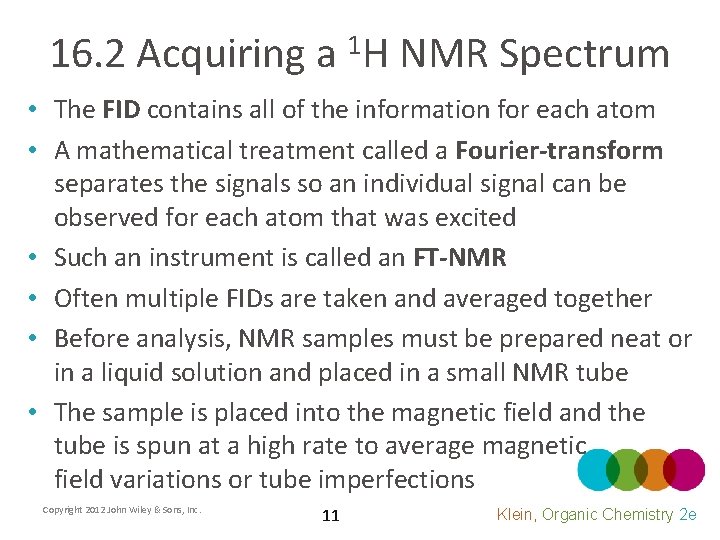
16. 2 Acquiring a 1 H NMR Spectrum • The FID contains all of the information for each atom • A mathematical treatment called a Fourier-transform separates the signals so an individual signal can be observed for each atom that was excited • Such an instrument is called an FT-NMR • Often multiple FIDs are taken and averaged together • Before analysis, NMR samples must be prepared neat or in a liquid solution and placed in a small NMR tube • The sample is placed into the magnetic field and the tube is spun at a high rate to average magnetic field variations or tube imperfections Copyright 2012 John Wiley & Sons, Inc. 11 Klein, Organic Chemistry 2 e

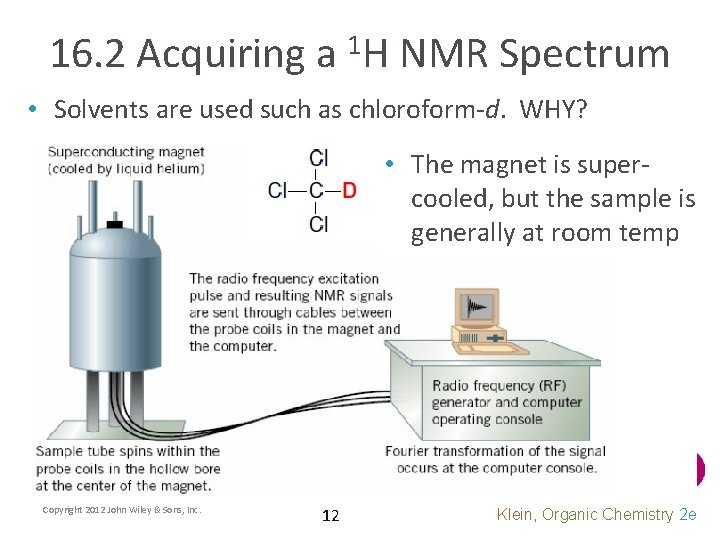
16. 2 Acquiring a 1 H NMR Spectrum • Solvents are used such as chloroform-d. WHY? • The magnet is supercooled, but the sample is generally at room temp Copyright 2012 John Wiley & Sons, Inc. 12 Klein, Organic Chemistry 2 e

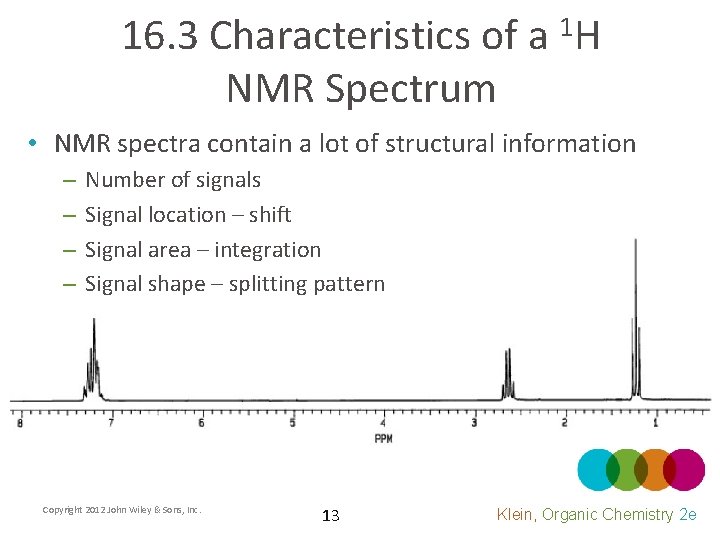
16. 3 Characteristics of a NMR Spectrum 1 H • NMR spectra contain a lot of structural information – – Number of signals Signal location – shift Signal area – integration Signal shape – splitting pattern Copyright 2012 John Wiley & Sons, Inc. 13 Klein, Organic Chemistry 2 e

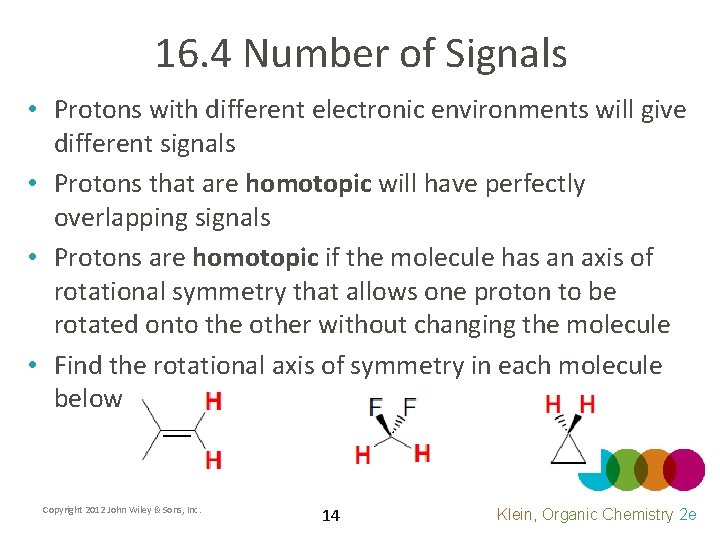
16. 4 Number of Signals • Protons with different electronic environments will give different signals • Protons that are homotopic will have perfectly overlapping signals • Protons are homotopic if the molecule has an axis of rotational symmetry that allows one proton to be rotated onto the other without changing the molecule • Find the rotational axis of symmetry in each molecule below Copyright 2012 John Wiley & Sons, Inc. 14 Klein, Organic Chemistry 2 e

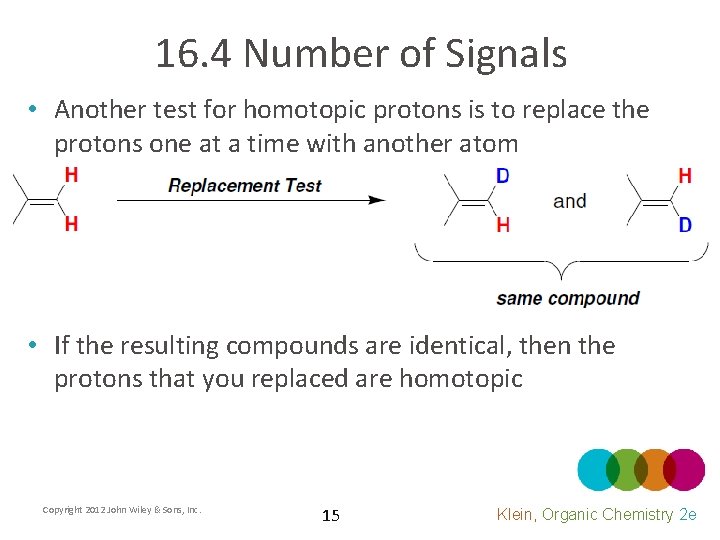
16. 4 Number of Signals • Another test for homotopic protons is to replace the protons one at a time with another atom • If the resulting compounds are identical, then the protons that you replaced are homotopic Copyright 2012 John Wiley & Sons, Inc. 15 Klein, Organic Chemistry 2 e

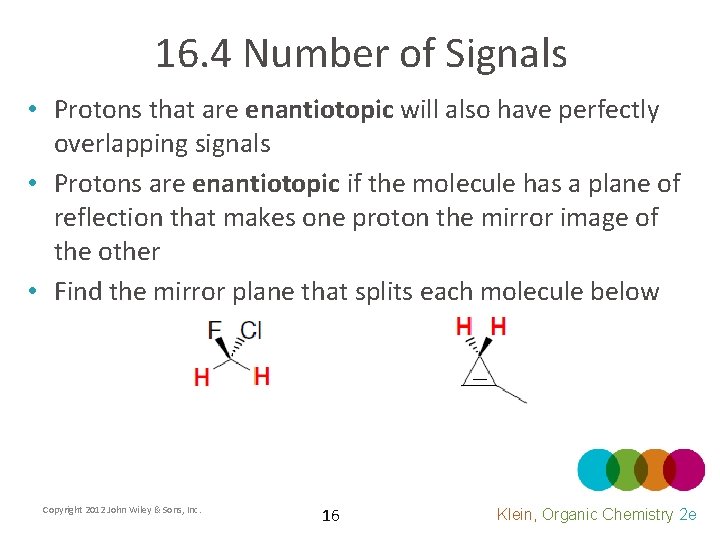
16. 4 Number of Signals • Protons that are enantiotopic will also have perfectly overlapping signals • Protons are enantiotopic if the molecule has a plane of reflection that makes one proton the mirror image of the other • Find the mirror plane that splits each molecule below Copyright 2012 John Wiley & Sons, Inc. 16 Klein, Organic Chemistry 2 e

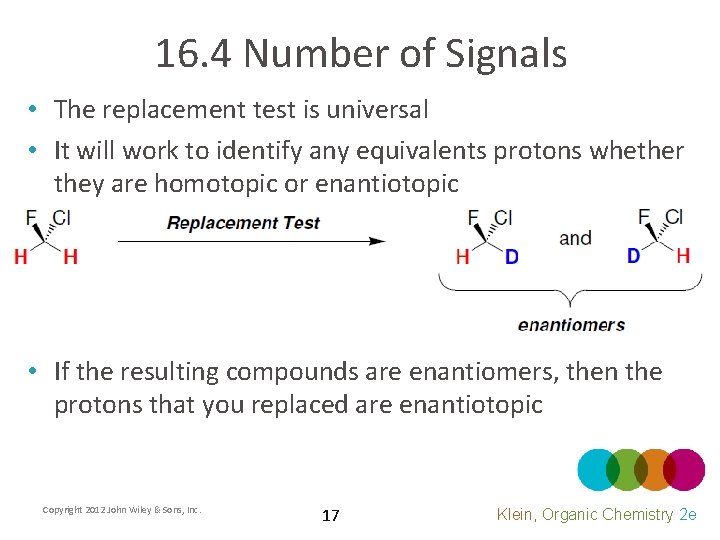
16. 4 Number of Signals • The replacement test is universal • It will work to identify any equivalents protons whether they are homotopic or enantiotopic • If the resulting compounds are enantiomers, then the protons that you replaced are enantiotopic Copyright 2012 John Wiley & Sons, Inc. 17 Klein, Organic Chemistry 2 e

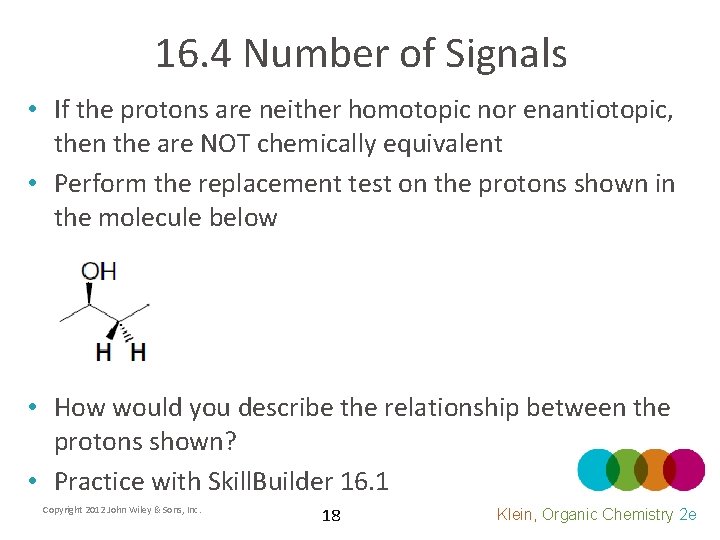
16. 4 Number of Signals • If the protons are neither homotopic nor enantiotopic, then the are NOT chemically equivalent • Perform the replacement test on the protons shown in the molecule below • How would you describe the relationship between the protons shown? • Practice with Skill. Builder 16. 1 Copyright 2012 John Wiley & Sons, Inc. 18 Klein, Organic Chemistry 2 e

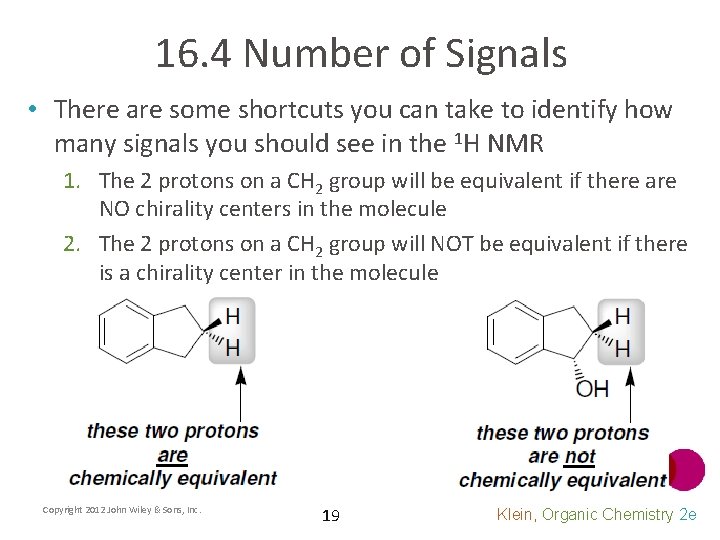
16. 4 Number of Signals • There are some shortcuts you can take to identify how many signals you should see in the 1 H NMR 1. The 2 protons on a CH 2 group will be equivalent if there are NO chirality centers in the molecule 2. The 2 protons on a CH 2 group will NOT be equivalent if there is a chirality center in the molecule Copyright 2012 John Wiley & Sons, Inc. 19 Klein, Organic Chemistry 2 e

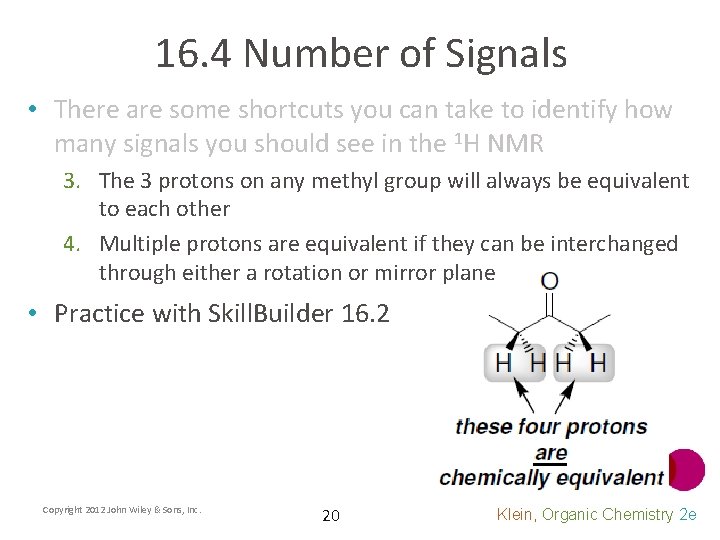
16. 4 Number of Signals • There are some shortcuts you can take to identify how many signals you should see in the 1 H NMR 3. The 3 protons on any methyl group will always be equivalent to each other 4. Multiple protons are equivalent if they can be interchanged through either a rotation or mirror plane • Practice with Skill. Builder 16. 2 Copyright 2012 John Wiley & Sons, Inc. 20 Klein, Organic Chemistry 2 e

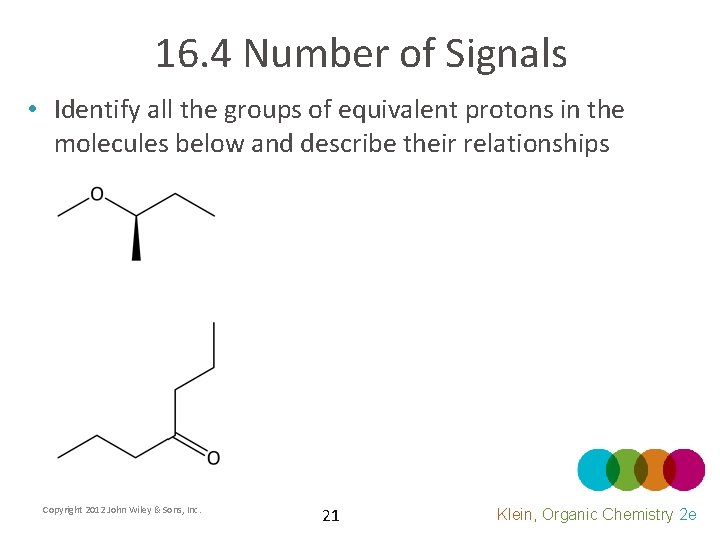
16. 4 Number of Signals • Identify all the groups of equivalent protons in the molecules below and describe their relationships Copyright 2012 John Wiley & Sons, Inc. 21 Klein, Organic Chemistry 2 e

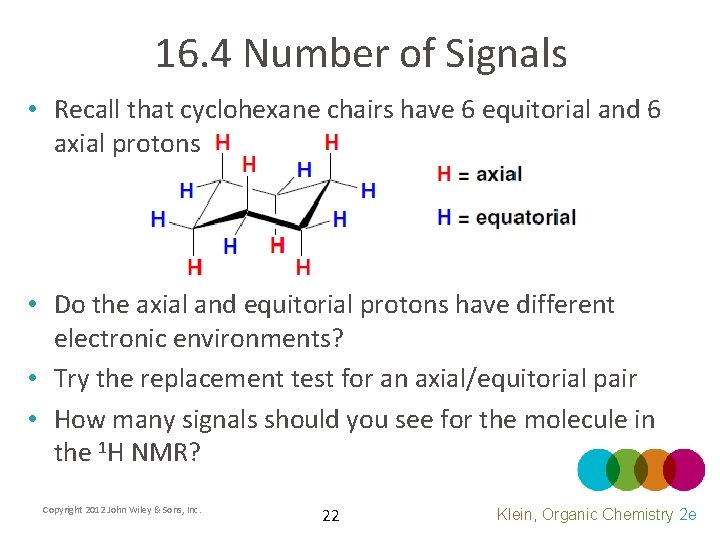
16. 4 Number of Signals • Recall that cyclohexane chairs have 6 equitorial and 6 axial protons • Do the axial and equitorial protons have different electronic environments? • Try the replacement test for an axial/equitorial pair • How many signals should you see for the molecule in the 1 H NMR? Copyright 2012 John Wiley & Sons, Inc. 22 Klein, Organic Chemistry 2 e

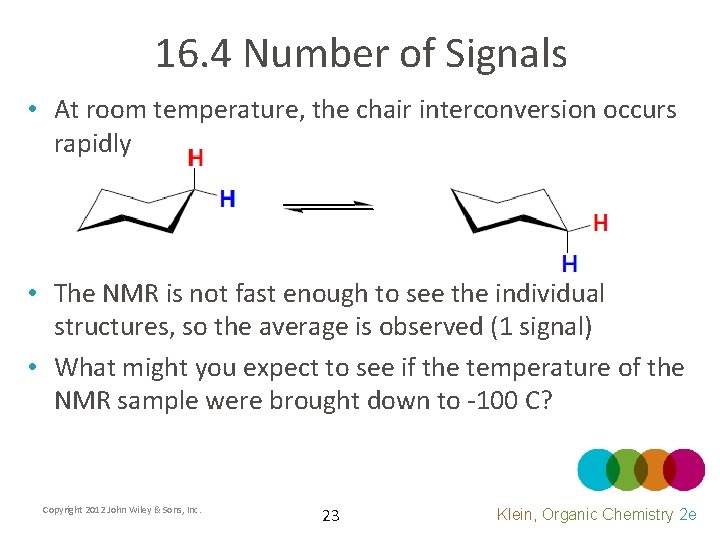
16. 4 Number of Signals • At room temperature, the chair interconversion occurs rapidly • The NMR is not fast enough to see the individual structures, so the average is observed (1 signal) • What might you expect to see if the temperature of the NMR sample were brought down to -100 C? Copyright 2012 John Wiley & Sons, Inc. 23 Klein, Organic Chemistry 2 e

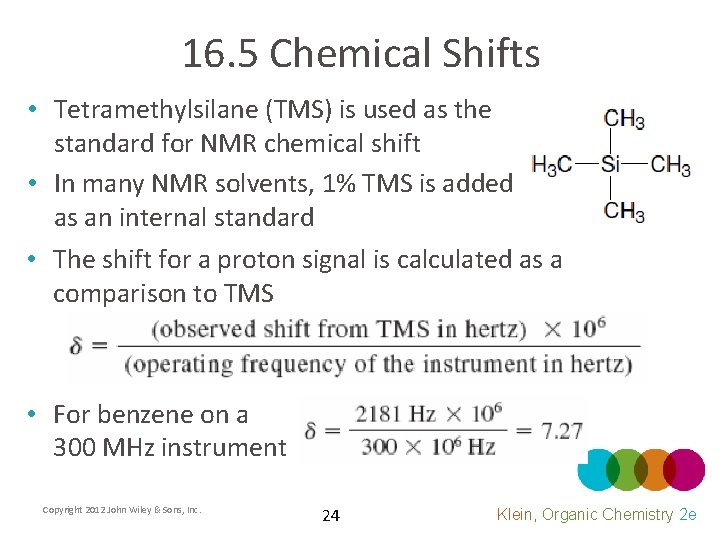
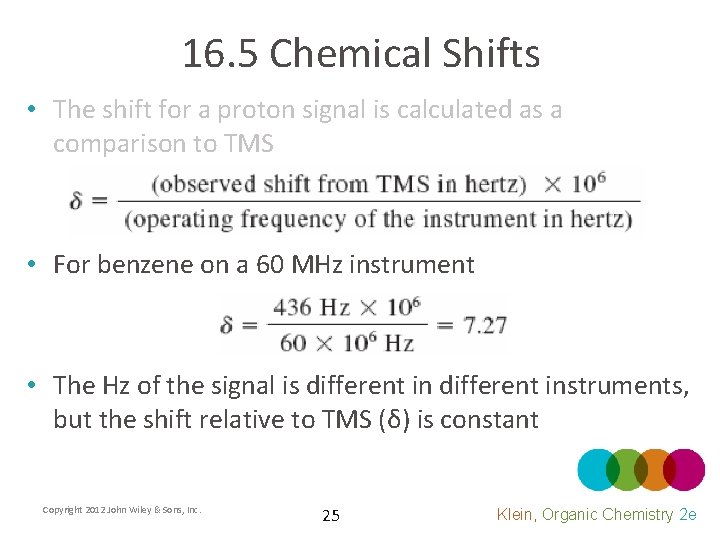
16. 5 Chemical Shifts • Tetramethylsilane (TMS) is used as the standard for NMR chemical shift • In many NMR solvents, 1% TMS is added as an internal standard • The shift for a proton signal is calculated as a comparison to TMS • For benzene on a 300 MHz instrument Copyright 2012 John Wiley & Sons, Inc. 24 Klein, Organic Chemistry 2 e

16. 5 Chemical Shifts • The shift for a proton signal is calculated as a comparison to TMS • For benzene on a 60 MHz instrument • The Hz of the signal is different instruments, but the shift relative to TMS (δ) is constant Copyright 2012 John Wiley & Sons, Inc. 25 Klein, Organic Chemistry 2 e

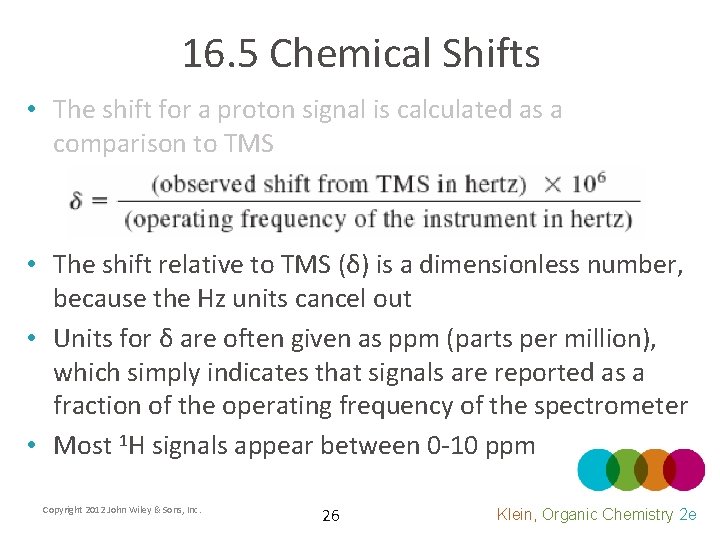
16. 5 Chemical Shifts • The shift for a proton signal is calculated as a comparison to TMS • The shift relative to TMS (δ) is a dimensionless number, because the Hz units cancel out • Units for δ are often given as ppm (parts per million), which simply indicates that signals are reported as a fraction of the operating frequency of the spectrometer • Most 1 H signals appear between 0 -10 ppm Copyright 2012 John Wiley & Sons, Inc. 26 Klein, Organic Chemistry 2 e

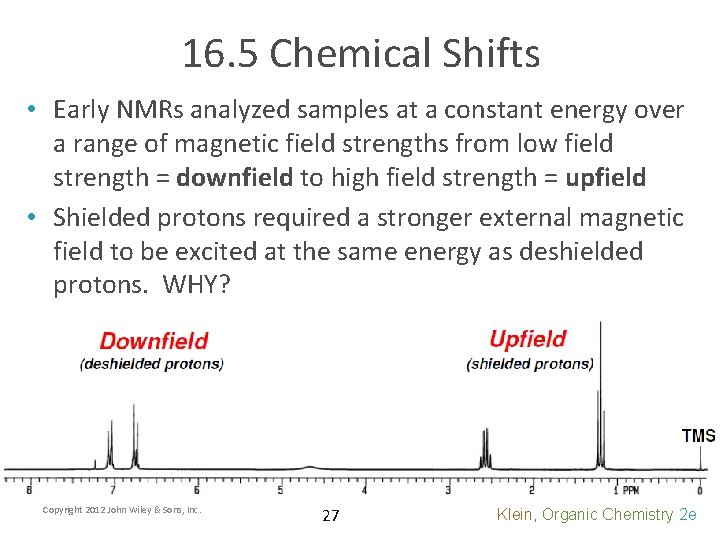
16. 5 Chemical Shifts • Early NMRs analyzed samples at a constant energy over a range of magnetic field strengths from low field strength = downfield to high field strength = upfield • Shielded protons required a stronger external magnetic field to be excited at the same energy as deshielded protons. WHY? Copyright 2012 John Wiley & Sons, Inc. 27 Klein, Organic Chemistry 2 e

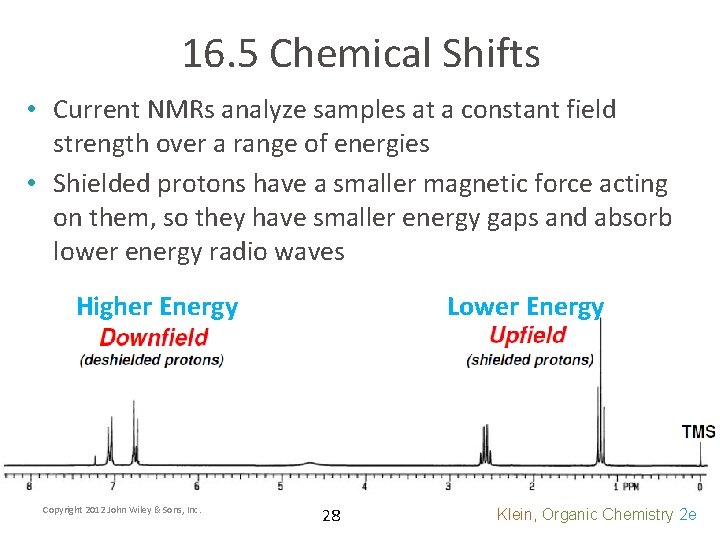
16. 5 Chemical Shifts • Current NMRs analyze samples at a constant field strength over a range of energies • Shielded protons have a smaller magnetic force acting on them, so they have smaller energy gaps and absorb lower energy radio waves Higher Energy Copyright 2012 John Wiley & Sons, Inc. Lower Energy 28 Klein, Organic Chemistry 2 e

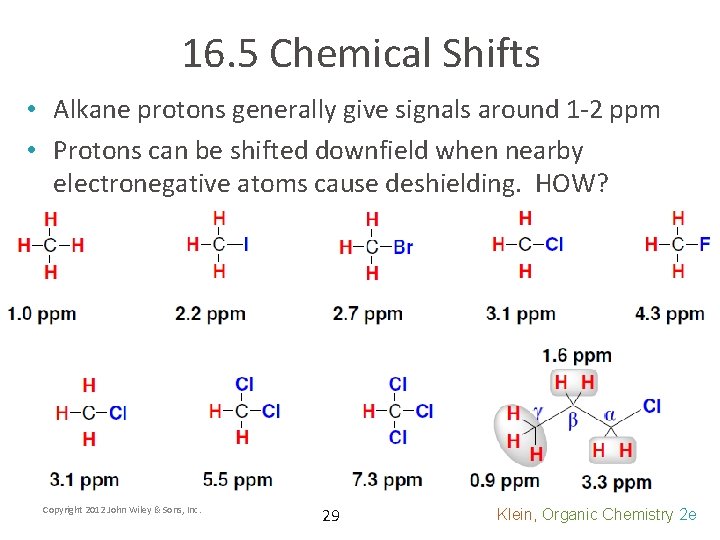
16. 5 Chemical Shifts • Alkane protons generally give signals around 1 -2 ppm • Protons can be shifted downfield when nearby electronegative atoms cause deshielding. HOW? Copyright 2012 John Wiley & Sons, Inc. 29 Klein, Organic Chemistry 2 e

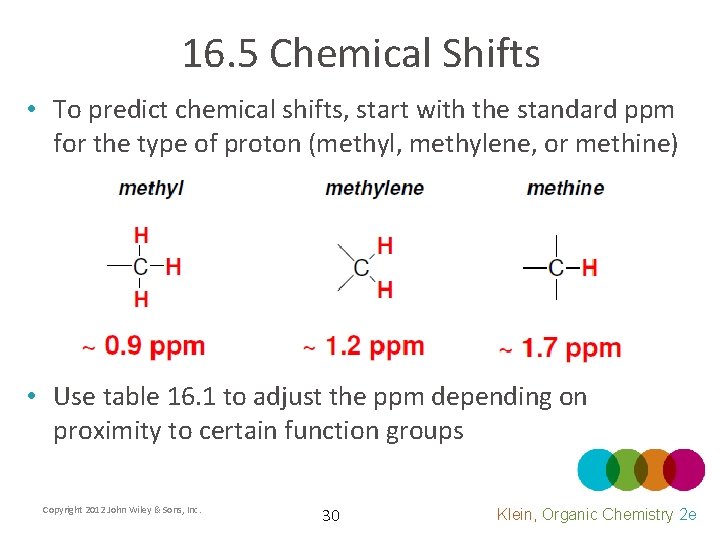
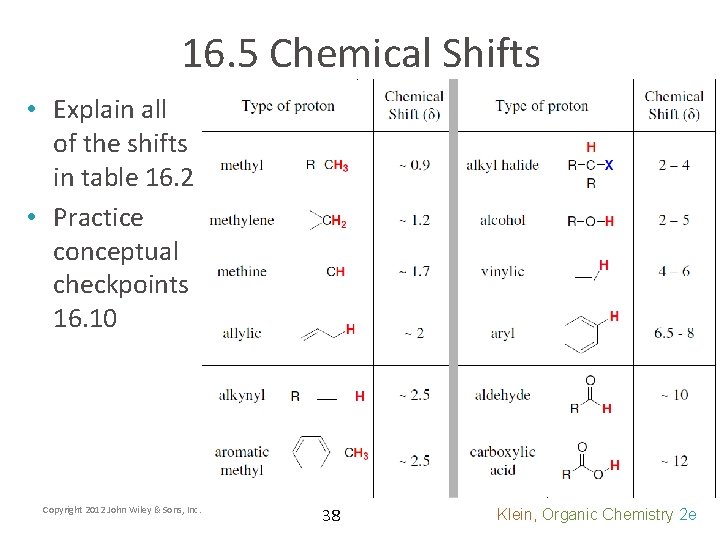
16. 5 Chemical Shifts • To predict chemical shifts, start with the standard ppm for the type of proton (methyl, methylene, or methine) • Use table 16. 1 to adjust the ppm depending on proximity to certain function groups Copyright 2012 John Wiley & Sons, Inc. 30 Klein, Organic Chemistry 2 e

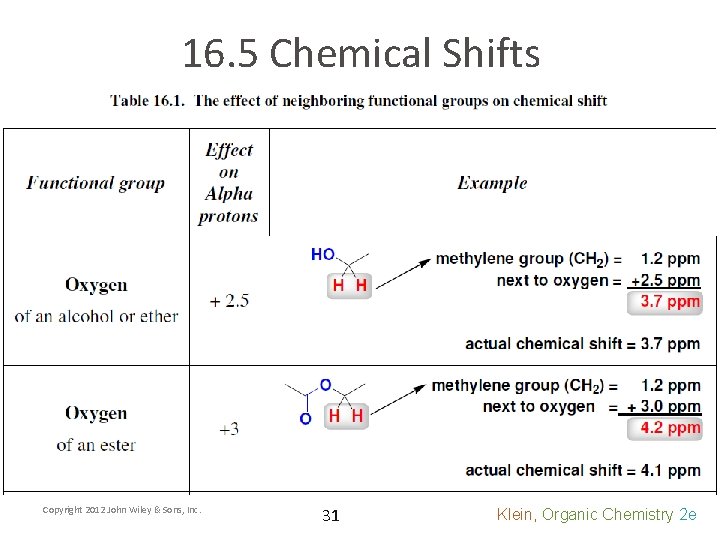
16. 5 Chemical Shifts Copyright 2012 John Wiley & Sons, Inc. 31 Klein, Organic Chemistry 2 e

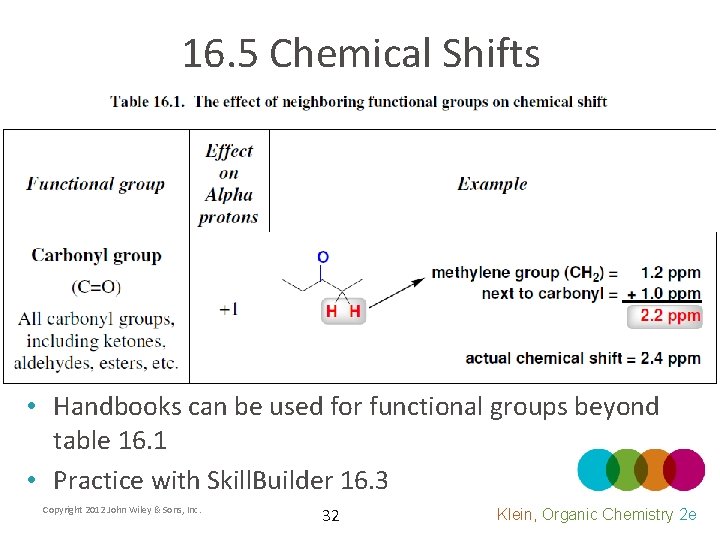
16. 5 Chemical Shifts • Handbooks can be used for functional groups beyond table 16. 1 • Practice with Skill. Builder 16. 3 Copyright 2012 John Wiley & Sons, Inc. 32 Klein, Organic Chemistry 2 e

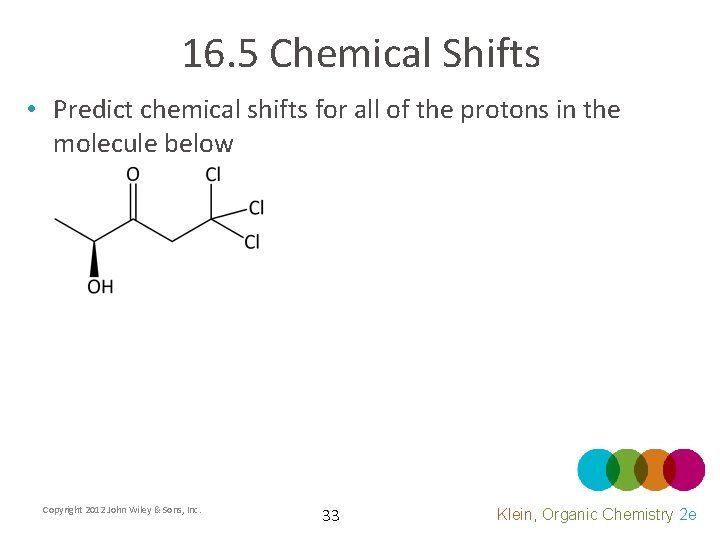
16. 5 Chemical Shifts • Predict chemical shifts for all of the protons in the molecule below Copyright 2012 John Wiley & Sons, Inc. 33 Klein, Organic Chemistry 2 e

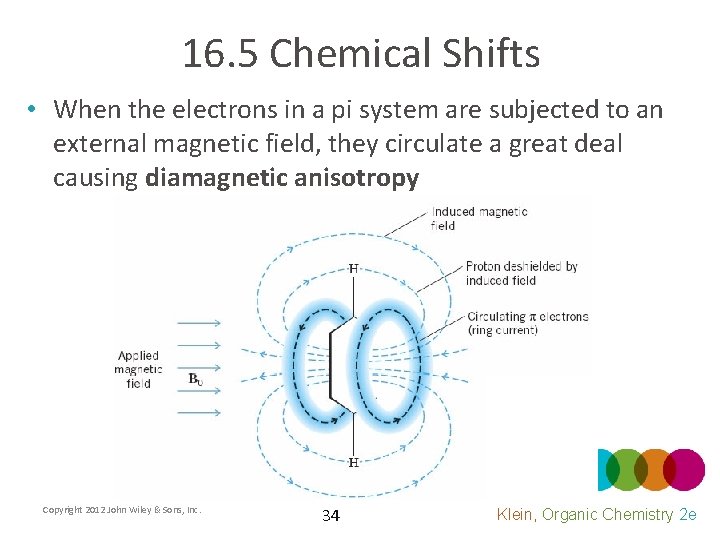
16. 5 Chemical Shifts • When the electrons in a pi system are subjected to an external magnetic field, they circulate a great deal causing diamagnetic anisotropy Copyright 2012 John Wiley & Sons, Inc. 34 Klein, Organic Chemistry 2 e

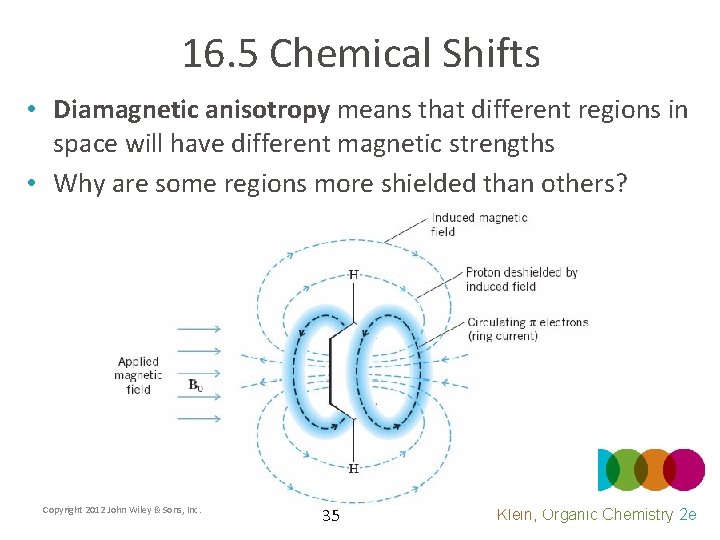
16. 5 Chemical Shifts • Diamagnetic anisotropy means that different regions in space will have different magnetic strengths • Why are some regions more shielded than others? Copyright 2012 John Wiley & Sons, Inc. 35 Klein, Organic Chemistry 2 e

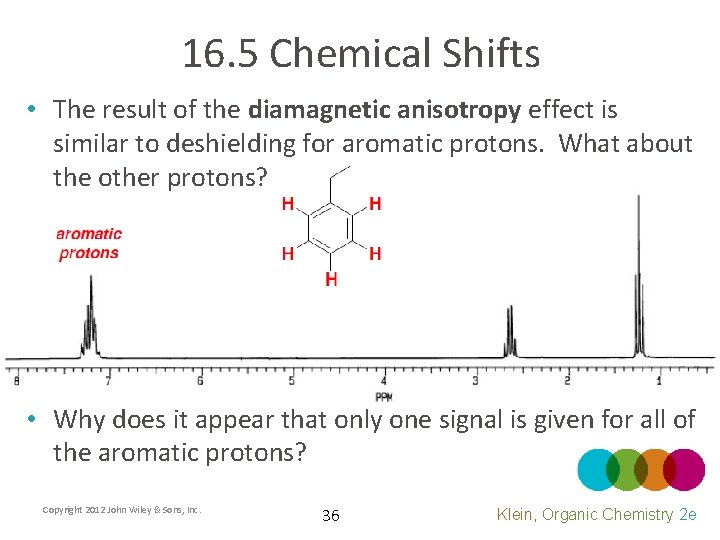
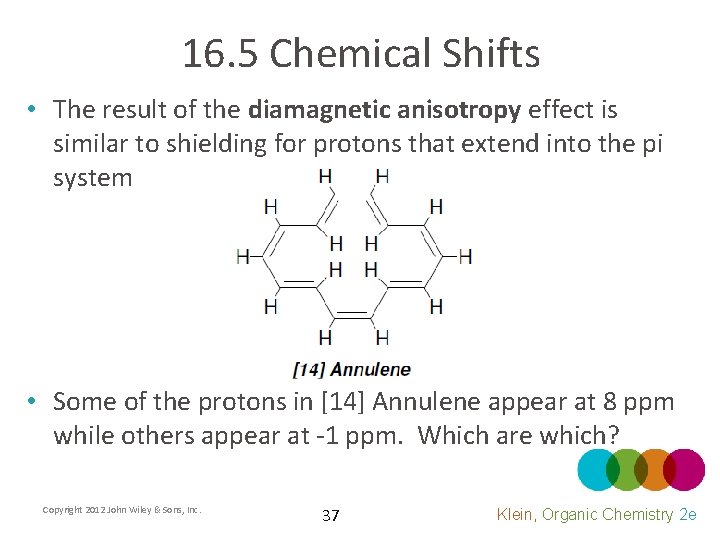
16. 5 Chemical Shifts • The result of the diamagnetic anisotropy effect is similar to deshielding for aromatic protons. What about the other protons? • Why does it appear that only one signal is given for all of the aromatic protons? Copyright 2012 John Wiley & Sons, Inc. 36 Klein, Organic Chemistry 2 e

16. 5 Chemical Shifts • The result of the diamagnetic anisotropy effect is similar to shielding for protons that extend into the pi system • Some of the protons in [14] Annulene appear at 8 ppm while others appear at -1 ppm. Which are which? Copyright 2012 John Wiley & Sons, Inc. 37 Klein, Organic Chemistry 2 e

16. 5 Chemical Shifts • Explain all of the shifts in table 16. 2 • Practice conceptual checkpoints 16. 10 Copyright 2012 John Wiley & Sons, Inc. 38 Klein, Organic Chemistry 2 e

Study Guide for sections 16. 1 -16. 5 DAY 22, Terms to know: Sections 16. 1 -16. 5 Nuclear magnetic resonance, magnetic moment, alpha spin state, beta spin state, shielded, deshielded, resonance (in regard to spin states), free induction decay (FID), Fourier-transform (FT), chemical shift DAY 22, Specific outcomes and skills that may be tested on exam 3: Sections 16. 1 -16. 5 • Be able to explain what happens to magnets and also the spin states of nuclei when atoms are placed in a magnetic field. • Be able to explain what happens to the spin states when radio waves of the correct frequency excite nuclei in a magnetic field. • Be able to explain why shielded atoms have smaller energy gaps between alpha and beta spin states. • Be able to explain the relationship between the strength of the magnetic field the size of the energy gap between spin states. • Be able to explain how modern NMR instruments are used to obtain a FID. • Be able to explain how and why NMR samples are prepared including state of matter, solvent, spinning. • Be able to determine when H atoms are equivalent or non equivalent. • Be able to identify homotopic, enantiotopic, and diastereotopic protons and explain how such protons affect the number of signals and splitting patterns in the proton NMR. • Be able to explain why TMS is added to many NMR samples. • Be able to explain and use the terms upfield, downfield, shielded, deshielded, higher and lower energy and frequency and explain the relationships such as upfield is the area where shielded nuclei signals appear, etc. • Be able to explain how electronegative atoms affect shielding and shifts of proton signals in NMR. • Be able to give approximate predictions for where on the ppm scale proton signals will appear given the structure of a molecule. • Be able to explain how aromatic rings affect the shifts of protons nearby. 39 Klein, Organic. Chemistry 2 e 2 e Klein, Organic

Practice Problems for sections 16. 1 -16. 5 Complete these problems outside of class until you are confident you have learned the SKILLS in this section outlined on the study guide and we will review some of them next class period. 16. 1 16. 2 16. 3 16. 4 16. 5 16. 7 16. 9 16. 10 16. 31 16. 34 40 Klein, Organic. Chemistry 2 e 2 e Klein, Organic

Prep for Day 23 Must Watch videos: https: //www. youtube. com/watch? v=6 d. P-m. NDn. U-A (1 H NMR integration) https: //www. youtube. com/watch? v=86 i. NZa. Z 0 S 84 (n+1 rule) https: //www. youtube. com/watch? v=w. ZJ_i. Axv. HGU (coupling constants) https: //www. youtube. com/watch? v=int. PEdg 4 Yyc (complex splitting) https: //www. youtube. com/watch? v=Yr. Hx 2 R 72 h. Fk (1 H NMR practice) Other helpful videos: https: //www. youtube. com/watch? v=Ql. M 21 sl-TDg (splitting) https: //www. youtube. com/watch? v=g. Zu 9 Hhz 23 EY and https: //www. youtube. com/watch? v=15 fz 6 Wo 84 IA (more 1 H NMR practice) http: //ps. uci. edu/content/chem-51 b-organic-chemistry (lecture 19) Read sections 16. 6 -16. 11 41 Klein, Organic. Chemistry 2 e 2 e Klein, Organic