15 8 NOTES Altering Fuels Altering Fuels The







- Slides: 14

15. 8 – NOTES Altering Fuels

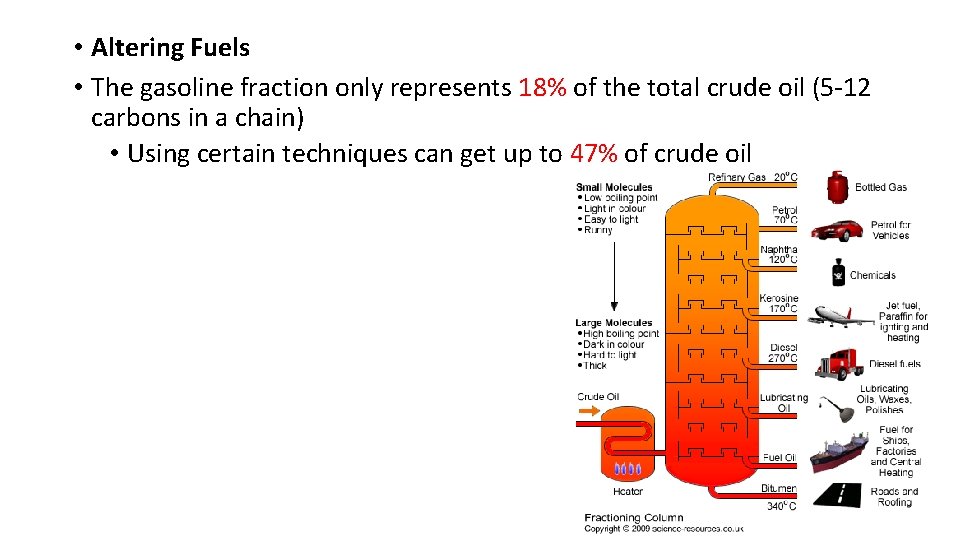
• Altering Fuels • The gasoline fraction only represents 18% of the total crude oil (5 12 carbons in a chain) • Using certain techniques can get up to 47% of crude oil

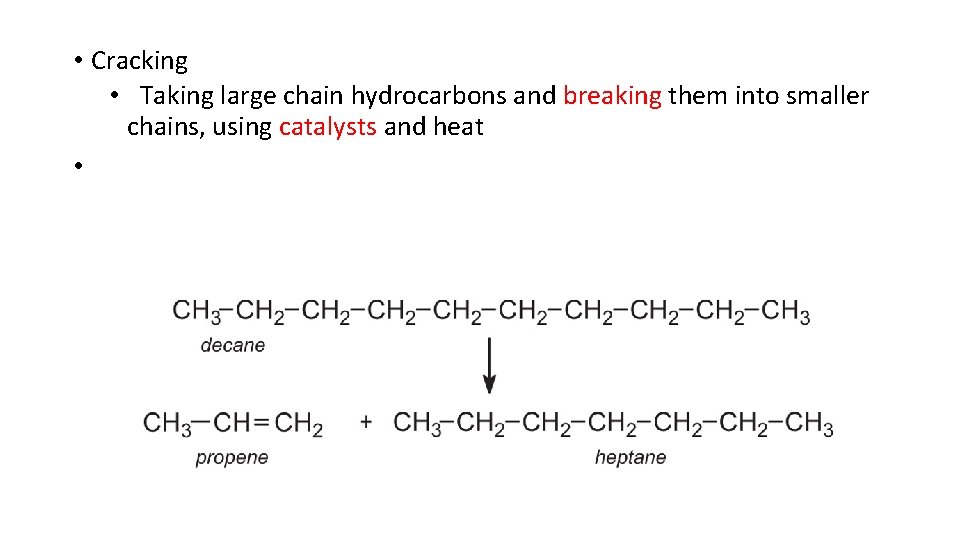
• Cracking • Taking large chain hydrocarbons and breaking them into smaller chains, using catalysts and heat •

• Catalyst • Something that increases the speed of a reaction and lowers the temperature needed to start the reaction • They are not used in the reactions – they are not part of the product • • Allows the burning of fuel to be cracked more efficiently because it takes place at a lower temperature (500 C rather than 700 C), which needs less energy

• A 16 carbon chain could be made into two 8 carbon chains • Can produce molecules of up to 14 carbons through cracking • C 5 C 12 are used in gasoline

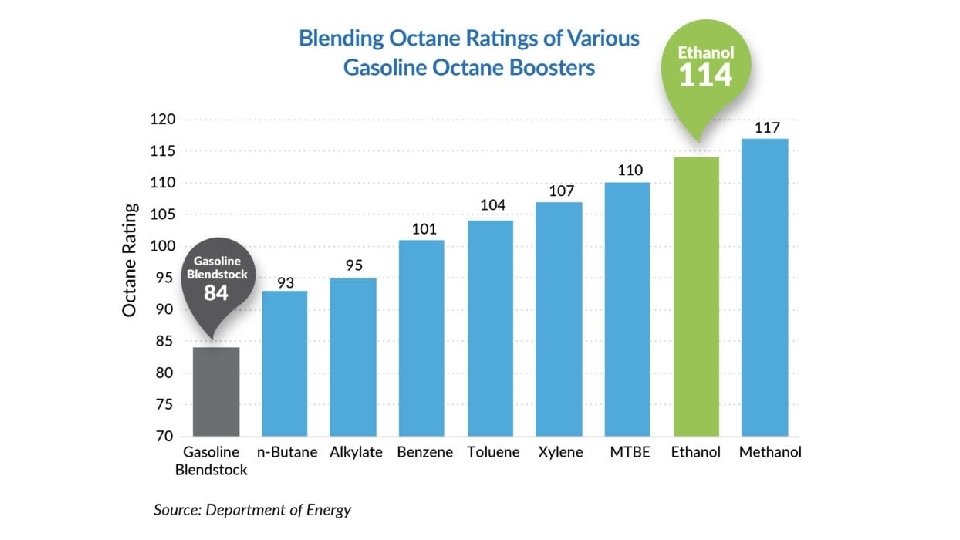
• Octane rating • Gas is made of mostly straight chain alkanes • Premature burning results in pinging/knocking • Branched chains don’t knock as much • • Octane rating tests efficiency • Free running and under a load and then the average is taken • Higher octane numbers result in better anti knock characteristics • • Prior to the 70 s, lead was added to increase octane rating • Tetraethyl lead ((C 2 H 4)4 Pb) • Why was lead banned from fuels? • Bad for the environment, can cause many health problems


• Oxygenated fuels • Fuels that have extra C, H and O to enhance octane • • Less energy is delivered, but the octane increases and reduces pollutants • • •

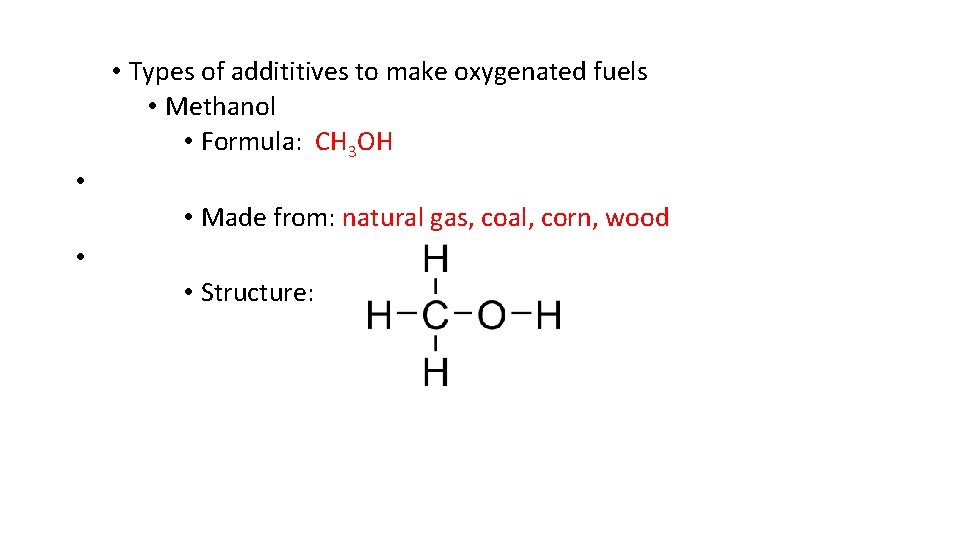
• Types of addititives to make oxygenated fuels • Methanol • Formula: CH 3 OH • • Made from: natural gas, coal, corn, wood • • Structure:

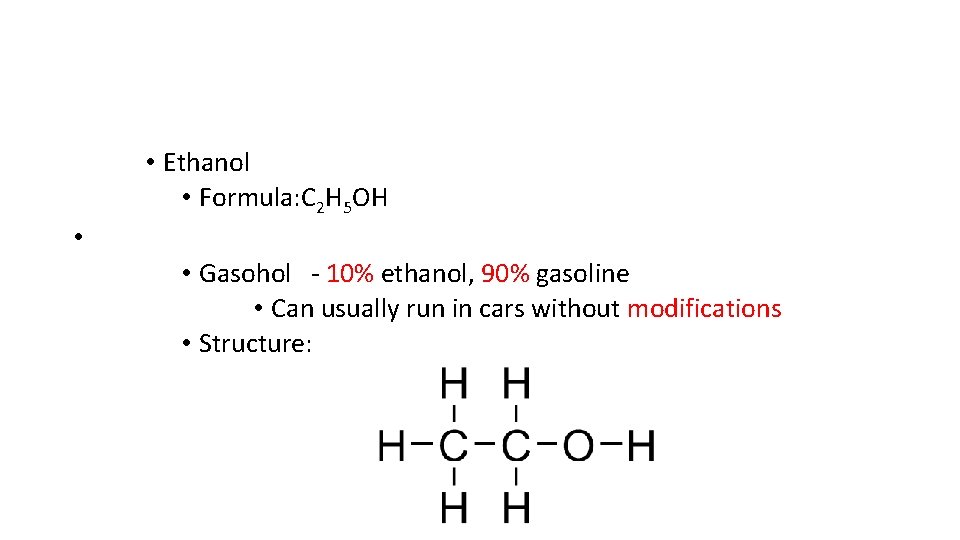
• Ethanol • Formula: C 2 H 5 OH • • Gasohol 10% ethanol, 90% gasoline • Can usually run in cars without modifications • Structure:

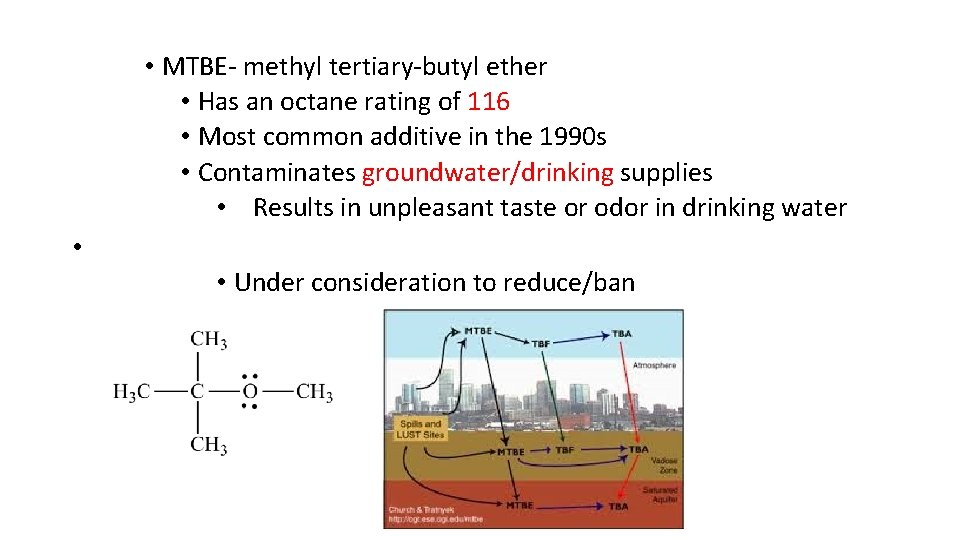
• MTBE methyl tertiary butyl ether • Has an octane rating of 116 • Most common additive in the 1990 s • Contaminates groundwater/drinking supplies • Results in unpleasant taste or odor in drinking water • • Under consideration to reduce/ban

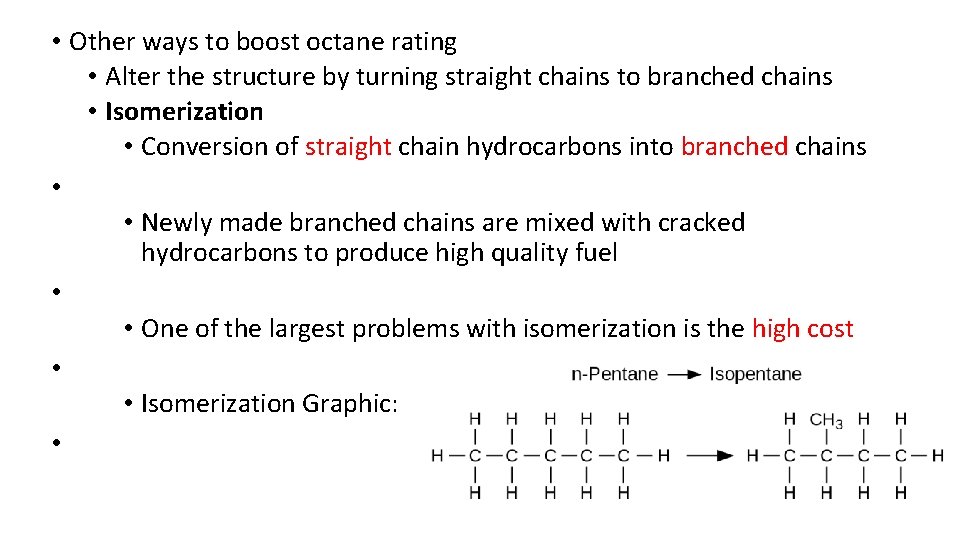
• Other ways to boost octane rating • Alter the structure by turning straight chains to branched chains • Isomerization • Conversion of straight chain hydrocarbons into branched chains • • Newly made branched chains are mixed with cracked hydrocarbons to produce high quality fuel • • One of the largest problems with isomerization is the high cost • • Isomerization Graphic: •

