15 3 Heterogeneous Aqueous Systems Chapter 15 Water





















- Slides: 37

15. 3 Heterogeneous Aqueous Systems > Chapter 15 Water and Aqueous Systems 15. 1 Water and Its Properties 15. 2 Homogeneous Aqueous Systems 15. 3 Heterogeneous Aqueous Systems 1 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

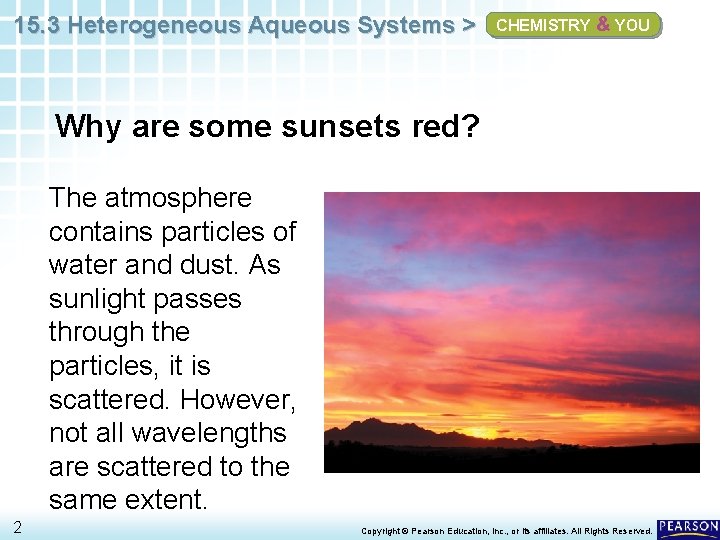
15. 3 Heterogeneous Aqueous Systems > CHEMISTRY & YOU Why are some sunsets red? The atmosphere contains particles of water and dust. As sunlight passes through the particles, it is scattered. However, not all wavelengths are scattered to the same extent. 2 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Suspensions What is the difference between a suspension and a solution? 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Suspensions What is the difference between a suspension and a solution? • A suspension is a mixture from which particles settle out upon standing. 4 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Suspensions A suspension differs from a solution because the particles of a suspension are much larger and do not stay suspended indefinitely. 5 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Suspensions A suspension differs from a solution because the particles of a suspension are much larger and do not stay suspended indefinitely. • The particles in a typical suspension have an average diameter greater than 1000 nm. • By contrast, the particle size in a solution is usually about 1 nm. 6 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Suspensions • A solution is a homogeneous mixture. • Suspensions are heterogeneous because at least two substances can be clearly identified. 7 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Suspensions The difference between a solution and suspension is easily seen when the type of mixture is filtered. The small size of the solute particles in a solution allows them to pass through filter paper. 8 The particles of a suspension can be removed by filtration. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Explain why a mixture of sand water can be separated by filtration, but a mixture of salt and water cannot. 9 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Explain why a mixture of sand water can be separated by filtration, but a mixture of salt and water cannot. A mixture of sand water is a suspension, and a mixture of salt and water is a solution. The particles in the sand mixture are much larger than the ions in the salt mixture. The sand particles are too large to pass through filter paper; the ions are not. 10 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Colloids What distinguishes a colloid from a suspension and a solution? 11 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Colloids A colloid is a heterogeneous mixture containing particles that range in size from 1 nm to 1000 nm. • The particles are spread, or dispersed, throughout the dispersion medium, which can be a solid, liquid, or gas. 12 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

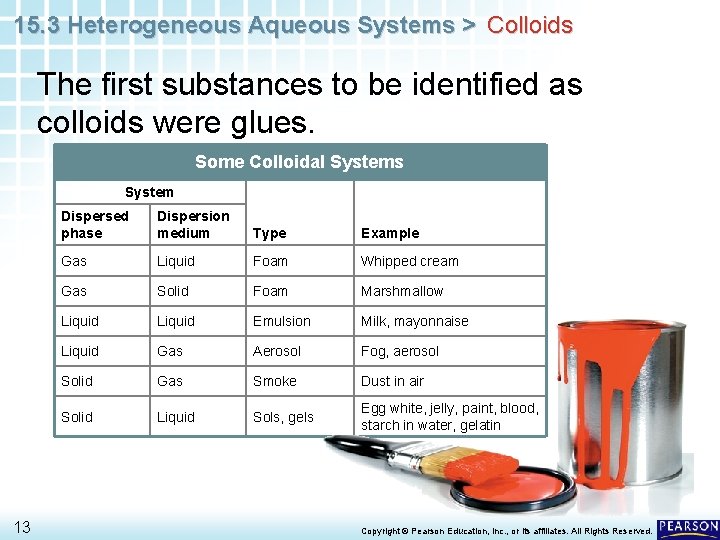
15. 3 Heterogeneous Aqueous Systems > Colloids The first substances to be identified as colloids were glues. Some Colloidal Systems System 13 Dispersed phase Dispersion medium Type Example Gas Liquid Foam Whipped cream Gas Solid Foam Marshmallow Liquid Emulsion Milk, mayonnaise Liquid Gas Aerosol Fog, aerosol Solid Gas Smoke Dust in air Solid Liquid Sols, gels Egg white, jelly, paint, blood, starch in water, gelatin Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Colloids have particles smaller than those in suspensions and larger than those in solutions. 14 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Colloids have particles smaller than those in suspensions and larger than those in solutions. • These intermediate-sized particles cannot be retained by filter paper as are the larger particles of a suspension. • They do not settle out with time. 15 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Colloids The Tyndall Effect You cannot see a beam of sunlight unless the light passes through particles of water (mist) or dust in the air. • These particles scatter the sunlight. • Similarly, a beam of light is visible as it passes through a colloid. 16 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

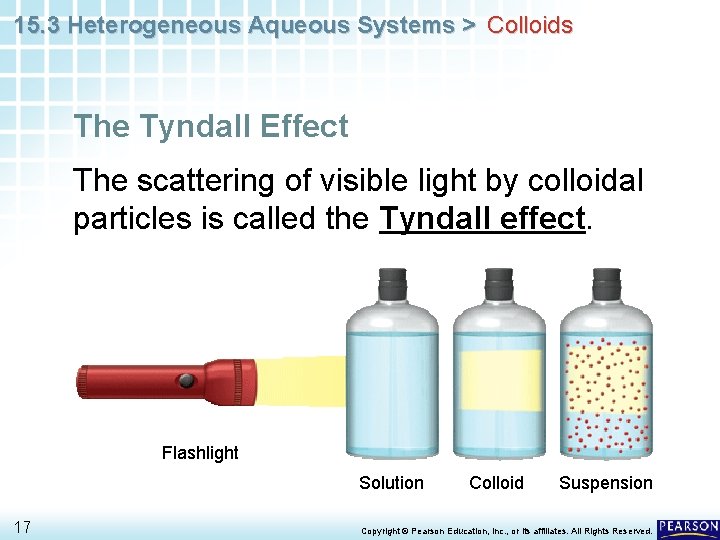
15. 3 Heterogeneous Aqueous Systems > Colloids The Tyndall Effect The scattering of visible light by colloidal particles is called the Tyndall effect. Flashlight Solution 17 Colloid Suspension Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

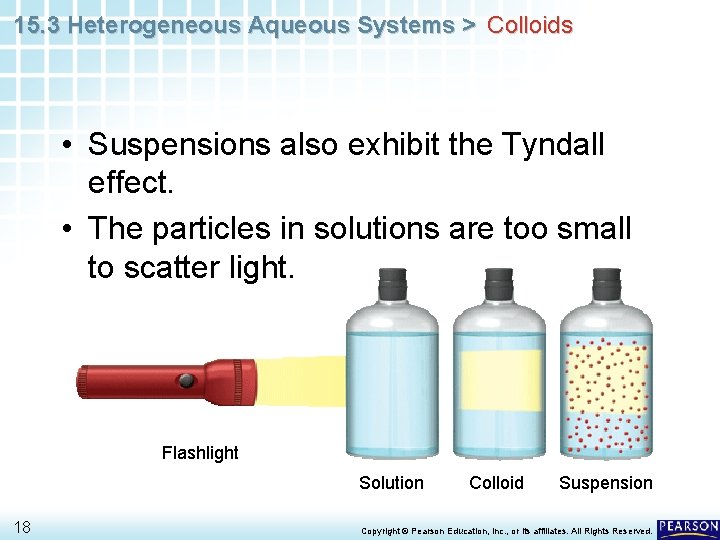
15. 3 Heterogeneous Aqueous Systems > Colloids • Suspensions also exhibit the Tyndall effect. • The particles in solutions are too small to scatter light. Flashlight Solution 18 Colloid Suspension Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > CHEMISTRY & YOU What would be the ideal conditions to see a red sunset? 19 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > CHEMISTRY & YOU What would be the ideal conditions to see a red sunset? A misty or foggy evening would be ideal for seeing a red sunset. There would be a large number of particles to scatter the sunlight. 20 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Colloids Brownian Motion Flashes of light, or scintillations, are seen when colloids are studied under a microscope. • Colloids scintillate because the particles reflecting and scattering the light move erratically. 21 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Colloids Brownian Motion The chaotic movement of colloidal particles, which was first observed by the Scottish botanist Robert Brown (1773– 1858), is called Brownian motion. 22 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Colloids Brownian Motion Brownian motion is caused by collisions of the molecules of the dispersion medium with the small, dispersed colloidal particles. • These collisions help prevent the colloidal particles from setting. 23 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Colloids Coagulation Colloidal particles also tend to stay suspended because they become charged by adsorbing ions from the dispersing medium onto their surface. • Adsorption means to adhere to a surface. 24 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Colloids Coagulation All the colloidal particles in a particular colloidal system will have the same charge, although the colloidal system is neutral. • The repulsion between the like-charged particles prevents the particles from forming heavier aggregates that would have a greater tendency to settle out. 25 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Colloids Coagulation A colloidal system can be destroyed or coagulated by the addition of electrolytes. • The added ions neutralize the charged colloidal particles. • The particles can clump together to form heavier aggregates and settle out from the dispersion. 26 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Colloids Emulsions An emulsion is a colloidal dispersion of a liquid in a liquid. 27 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Colloids Emulsions An emulsion is a colloidal dispersion of a liquid in a liquid. • An emulsifying agent is essential for the formation of an emulsion and for maintaining the emulsion’s stability. 28 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Colloids Emulsions • Oils and greases are not soluble in water. • However, oils and greases readily form a colloidal dispersion if soap or detergent is added to the water. 29 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Colloids Emulsions • One end of a large soap or detergent molecule is polar and is attracted to water molecules. • The other end of the soap or detergent molecule is nonpolar and is soluble in oil or grease. • Soaps and other emulsifying agents thus allow the formation of colloidal dispersions between liquids that do not ordinarily mix. 30 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

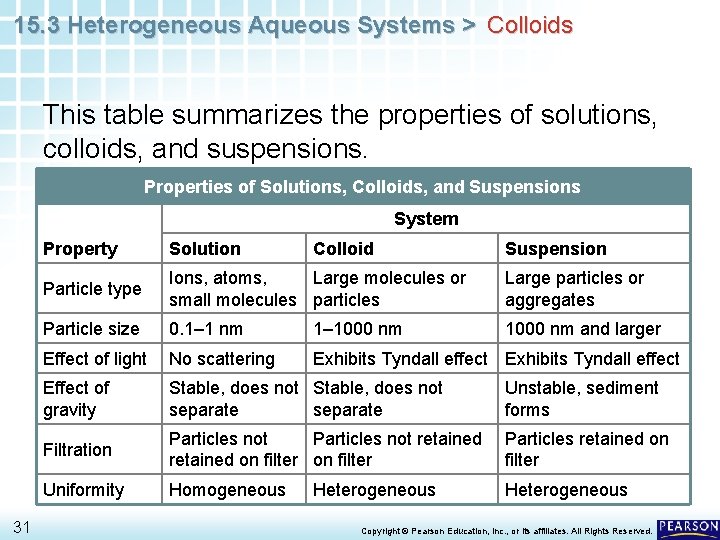
15. 3 Heterogeneous Aqueous Systems > Colloids This table summarizes the properties of solutions, colloids, and suspensions. Properties of Solutions, Colloids, and Suspensions System 31 Property Solution Colloid Particle type Ions, atoms, Large molecules or small molecules particles Large particles or aggregates Particle size 0. 1– 1 nm 1– 1000 nm and larger Effect of light No scattering Exhibits Tyndall effect Effect of gravity Stable, does not separate Unstable, sediment forms Filtration Particles not retained on filter Particles retained on filter Uniformity Homogeneous Heterogeneous Suspension Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Which of the following is a colloidal system? A. mud B. gasoline C. blood D. a mixture of sugar and water 32 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Which of the following is a colloidal system? A. mud B. gasoline C. blood D. a mixture of sugar and water 33 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Key Concepts A suspension differs from a solution because the particles of a suspension are much larger and do not stay suspended indefinitely. Colloids have particles smaller than those in suspensions and larger than those in solutions. 34 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Glossary Terms • suspension: a mixture from which some of the particles settle out slowly upon standing • colloid: a mixture whose particles are intermediate in size between those of a suspension and a solute solution • Tyndall effect: scattering of light by particles in a colloid or suspension, which causes a beam of light to become visible 35 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > Glossary Terms • Brownian motion: the chaotic movement of colloidal particles, caused by collision with particles of the solvent in which they are dispersed • emulsion: the colloidal dispersion of one liquid in another 36 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

15. 3 Heterogeneous Aqueous Systems > END OF 15. 3 37 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.