1411 Chapter 4 Aqueous Reactions and Solution Stoichiometry





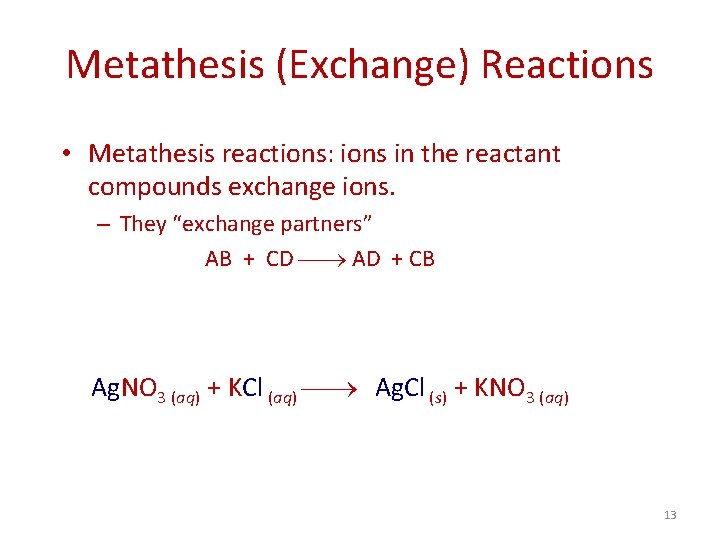
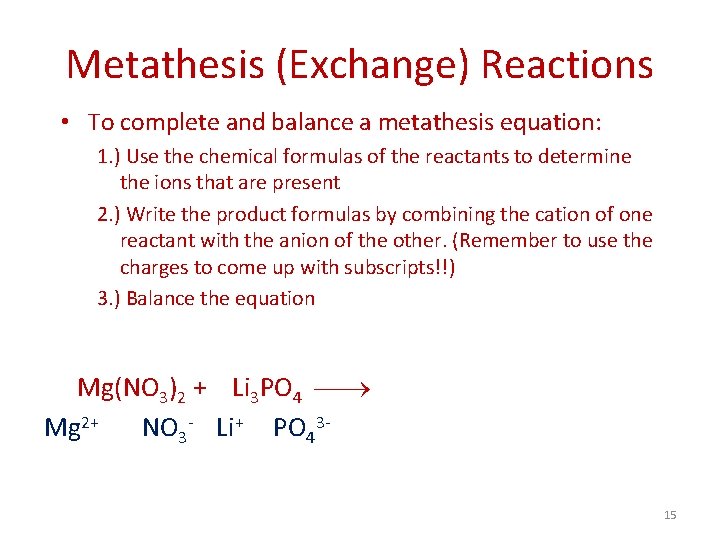








- Slides: 57

1411 Chapter 4 Aqueous Reactions and Solution Stoichiometry 1

Solutions • Solutions are defined as homogeneous mixtures of two or more pure substances. • Aqueous solution – solution in which water is the dissolving medium • The solvent is present in greatest abundance. • All other substances are solutes; they are dissolved in the solvent. – Example: Na. Cl dissolved in water: Na. Cl = solute water = solvent • Water can dissolve many ionic or molecular compounds – “universal solvent” 2

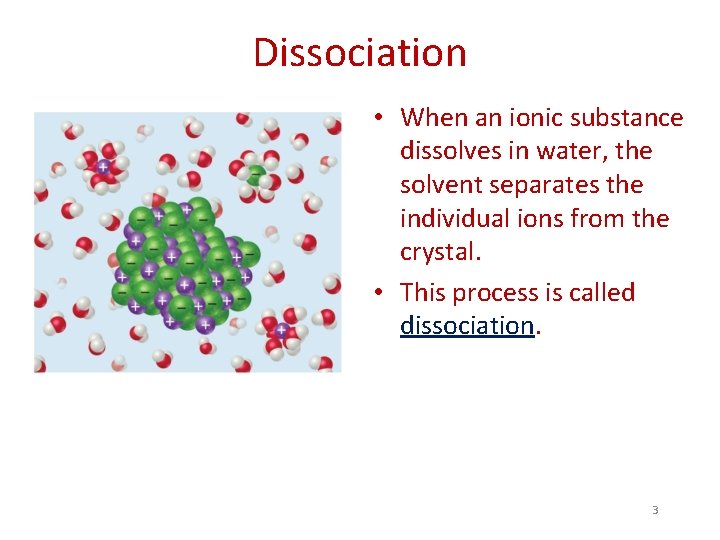
Dissociation • When an ionic substance dissolves in water, the solvent separates the individual ions from the crystal. • This process is called dissociation. 3

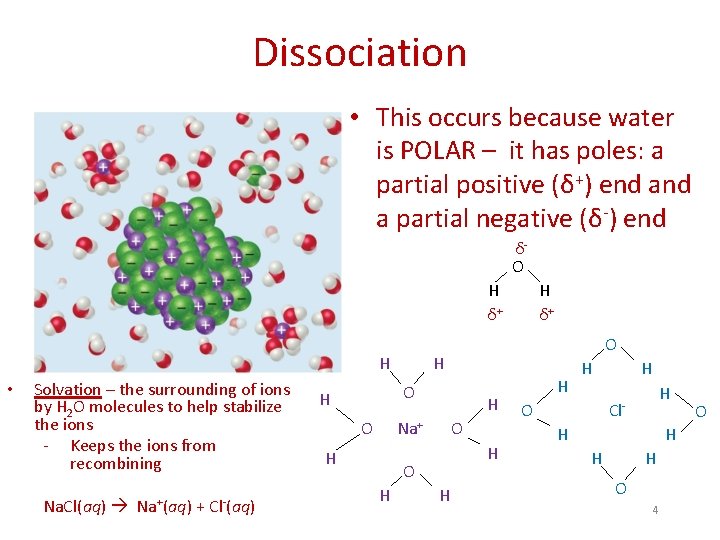
Dissociation • This occurs because water is POLAR – it has poles: a partial positive (δ+) end a partial negative (δ-) end δO H δ+ H • Solvation – the surrounding of ions by H 2 O molecules to help stabilize the ions - Keeps the ions from recombining Na. Cl(aq) Na+(aq) + Cl-(aq) O H O H Na+ H O H H δ+ H H H O H H Cl. H H O 4 O

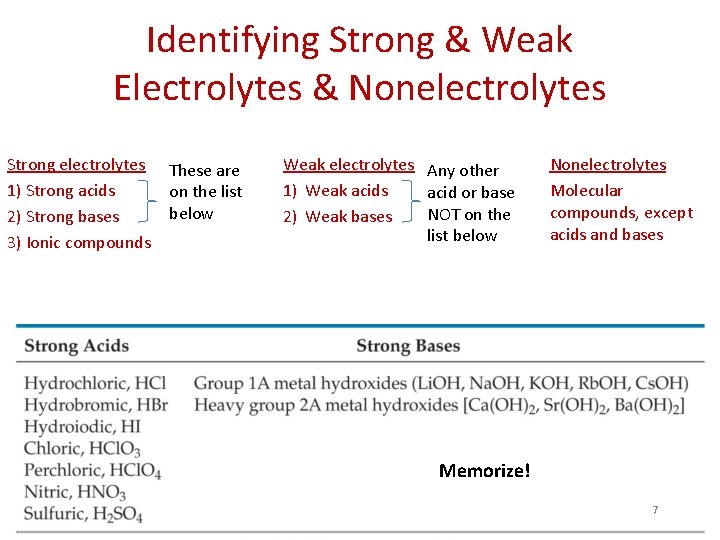
Electrolytes • An electrolyte is a substance that dissociates into ions when dissolved in water. – May be strong or weak • Strong electrolyte - completely dissociates into ions – Solutions conduct electricity well Ex: HCl(aq) H+(aq) + Cl-(aq) • Weak electrolyte – partially dissociates into ions, but some molecules remain intact – Solutions conduct electricity poorly Ex: CH 3 COOH(aq) CH 3 COO-(aq) + H+(aq) 5

Electrolytes • A nonelectrolyte may dissolve in water, but it does not dissociate into ions when it does so. – Solutions do not conduct electricity 6

Identifying Strong & Weak Electrolytes & Nonelectrolytes Strong electrolytes 1) Strong acids 2) Strong bases 3) Ionic compounds These are on the list below Weak electrolytes Any other 1) Weak acids acid or base NOT on the 2) Weak bases list below Nonelectrolytes Molecular compounds, except acids and bases Memorize! 7

Solution Chemistry • Pay attention to exactly what species are present in a reaction mixture (i. e. , solid, liquid, gas, aqueous solution). • If we are to understand reactivity, we must be aware of just what is changing during the course of a reaction… the driving force! – The driving force is what makes the reaction react (Ex: formation of a precipitate, gas, or liquid) – Without a driving force, the reaction won’t occur 8

Precipitation Reactions When one mixes ions that form compounds that are insoluble (as could be predicted by the solubility guidelines), a precipitate is formed. 9

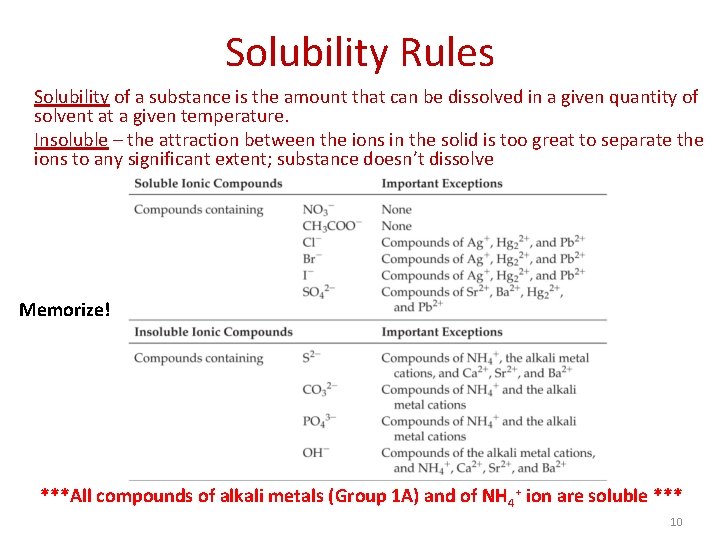
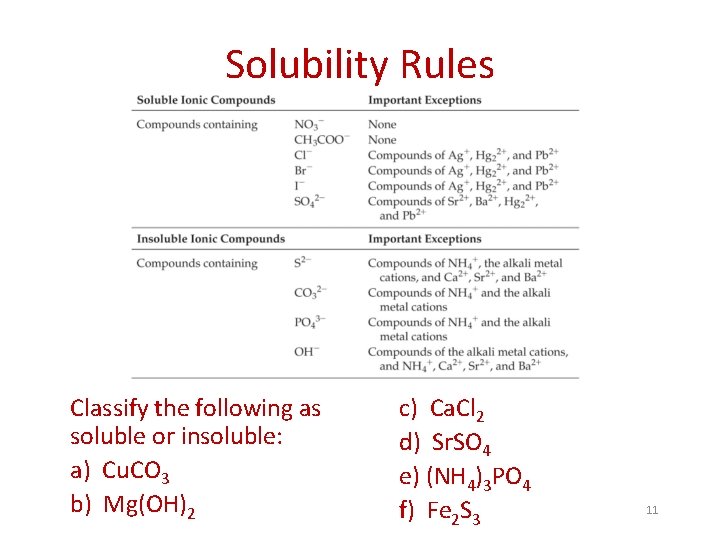
Solubility Rules Solubility of a substance is the amount that can be dissolved in a given quantity of solvent at a given temperature. Insoluble – the attraction between the ions in the solid is too great to separate the ions to any significant extent; substance doesn’t dissolve Memorize! ***All compounds of alkali metals (Group 1 A) and of NH 4+ ion are soluble *** 10

Solubility Rules Classify the following as soluble or insoluble: a) Cu. CO 3 b) Mg(OH)2 c) Ca. Cl 2 d) Sr. SO 4 e) (NH 4)3 PO 4 f) Fe 2 S 3 11

Electrolytic Properties ≠ Solubility Do not confuse extent of solubility with strong or weak electrolyte Examples: • Acetic acid (CH 3 COOH or HC 2 H 3 O 2): very soluble in water but weak electrolyte because most remains in form of molecule, not ions, in solution • Ba. Cl 2: not very soluble in water but strong electrolyte because what does dissolve dissociates completely 12
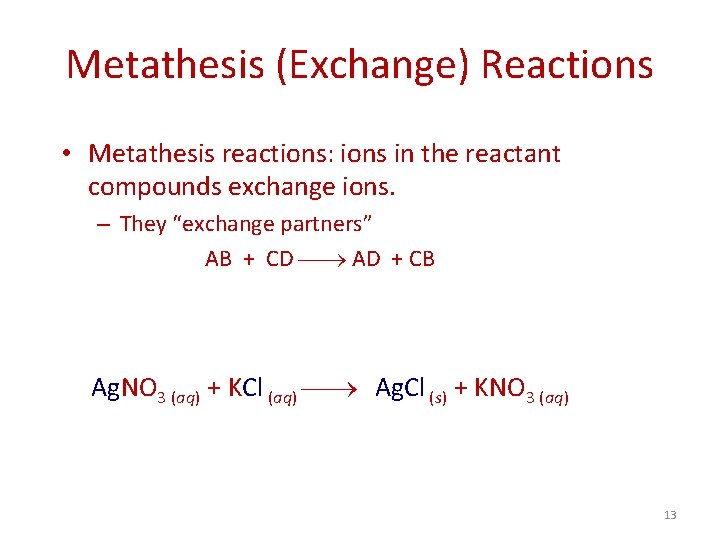
Metathesis (Exchange) Reactions • Metathesis reactions: ions in the reactant compounds exchange ions. – They “exchange partners” AB + CD AD + CB Ag. NO 3 (aq) + KCl (aq) Ag. Cl (s) + KNO 3 (aq) 13

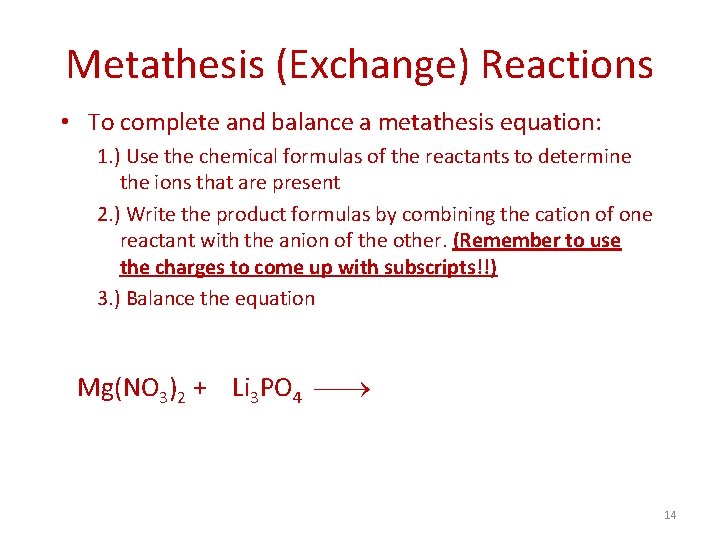
Metathesis (Exchange) Reactions • To complete and balance a metathesis equation: 1. ) Use the chemical formulas of the reactants to determine the ions that are present 2. ) Write the product formulas by combining the cation of one reactant with the anion of the other. (Remember to use the charges to come up with subscripts!!) 3. ) Balance the equation Mg(NO 3)2 + Li 3 PO 4 14
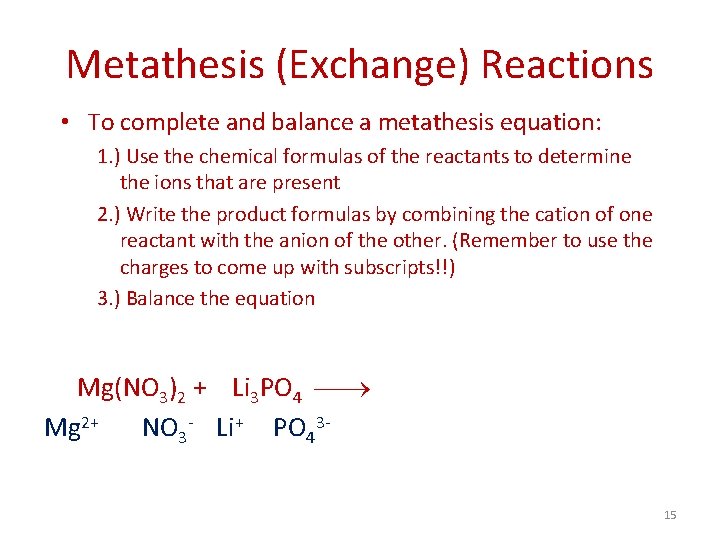
Metathesis (Exchange) Reactions • To complete and balance a metathesis equation: 1. ) Use the chemical formulas of the reactants to determine the ions that are present 2. ) Write the product formulas by combining the cation of one reactant with the anion of the other. (Remember to use the charges to come up with subscripts!!) 3. ) Balance the equation Mg(NO 3)2 + Li 3 PO 4 Mg 2+ NO 3 - Li+ PO 4315

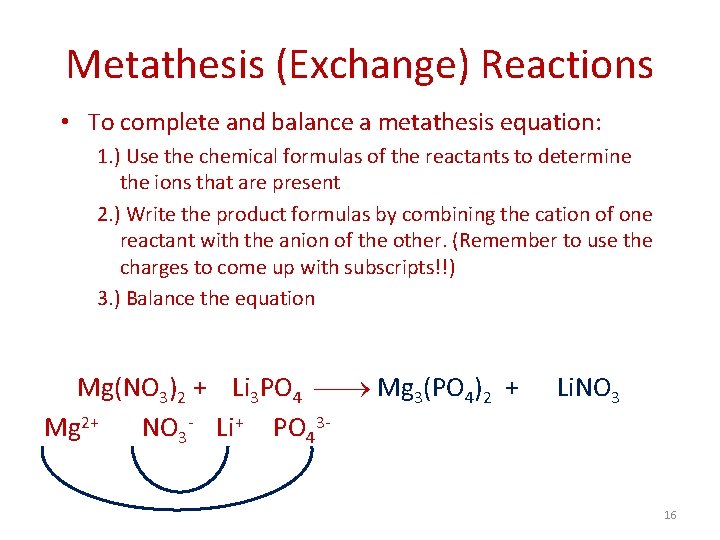
Metathesis (Exchange) Reactions • To complete and balance a metathesis equation: 1. ) Use the chemical formulas of the reactants to determine the ions that are present 2. ) Write the product formulas by combining the cation of one reactant with the anion of the other. (Remember to use the charges to come up with subscripts!!) 3. ) Balance the equation Mg(NO 3)2 + Li 3 PO 4 Mg 3(PO 4)2 + Mg 2+ NO 3 - Li+ PO 43 - Li. NO 3 16

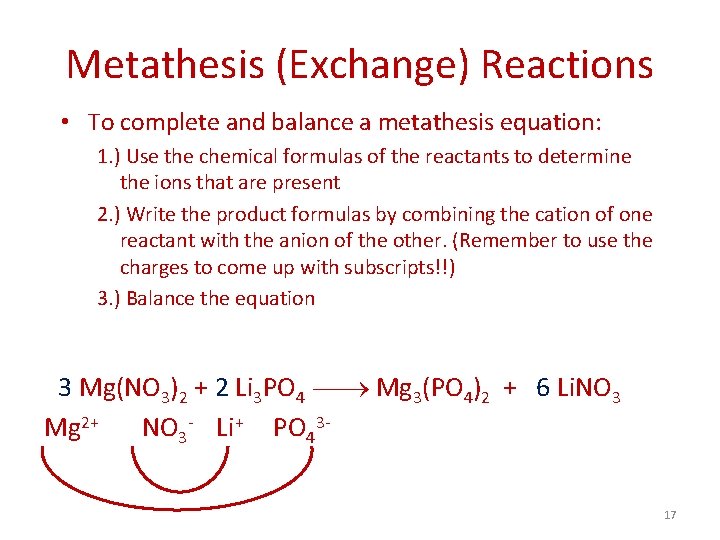
Metathesis (Exchange) Reactions • To complete and balance a metathesis equation: 1. ) Use the chemical formulas of the reactants to determine the ions that are present 2. ) Write the product formulas by combining the cation of one reactant with the anion of the other. (Remember to use the charges to come up with subscripts!!) 3. ) Balance the equation 3 Mg(NO 3)2 + 2 Li 3 PO 4 Mg 3(PO 4)2 + 6 Li. NO 3 Mg 2+ NO 3 - Li+ PO 4317

Predicting precipitation • To predict whether a precipitate forms when mixing aqueous solutions of 2 strong electrolytes: 1) Write the balanced metathesis reaction 2) Use solubility rules to determine if any of the products are insoluble 18

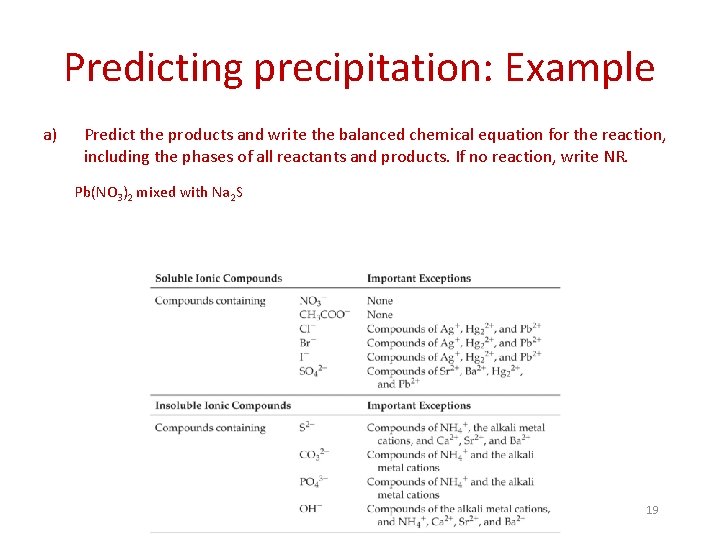
Predicting precipitation: Example a) Predict the products and write the balanced chemical equation for the reaction, including the phases of all reactants and products. If no reaction, write NR. Pb(NO 3)2 mixed with Na 2 S 19

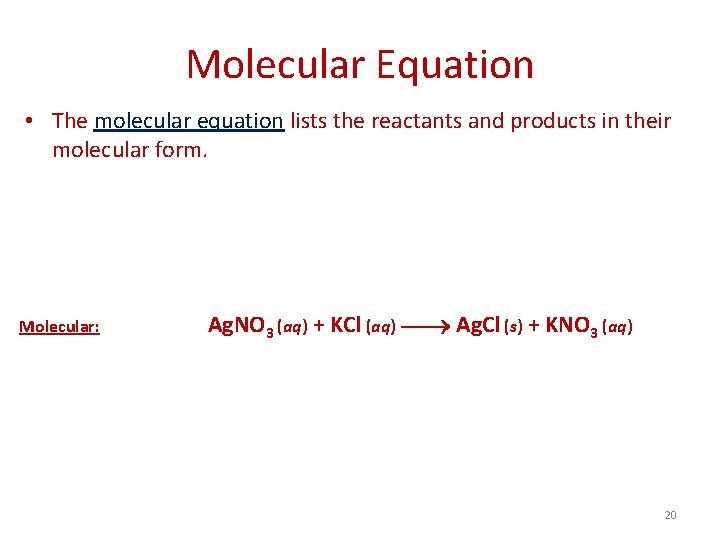
Molecular Equation • The molecular equation lists the reactants and products in their molecular form. Molecular: Ag. NO 3 (aq) + KCl (aq) Ag. Cl (s) + KNO 3 (aq) 20

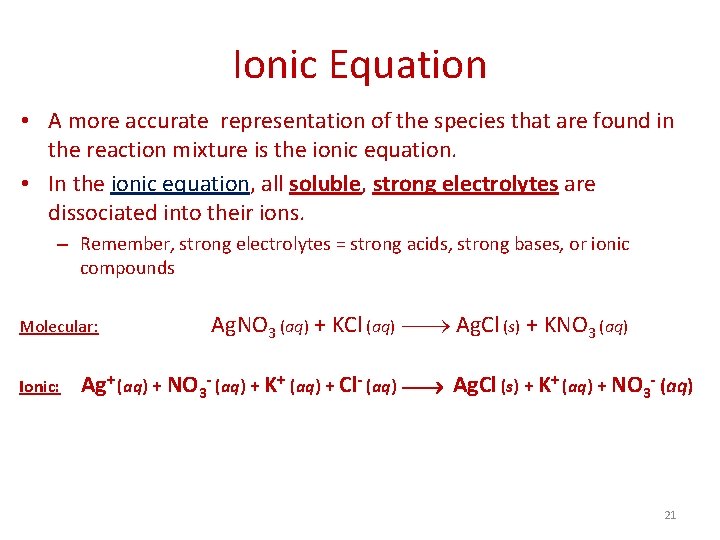
Ionic Equation • A more accurate representation of the species that are found in the reaction mixture is the ionic equation. • In the ionic equation, all soluble, strong electrolytes are dissociated into their ions. – Remember, strong electrolytes = strong acids, strong bases, or ionic compounds Molecular: Ionic: Ag. NO 3 (aq) + KCl (aq) Ag. Cl (s) + KNO 3 (aq) Ag+ (aq) + NO 3 - (aq) + K+ (aq) + Cl- (aq) Ag. Cl (s) + K+ (aq) + NO 3 - (aq) 21

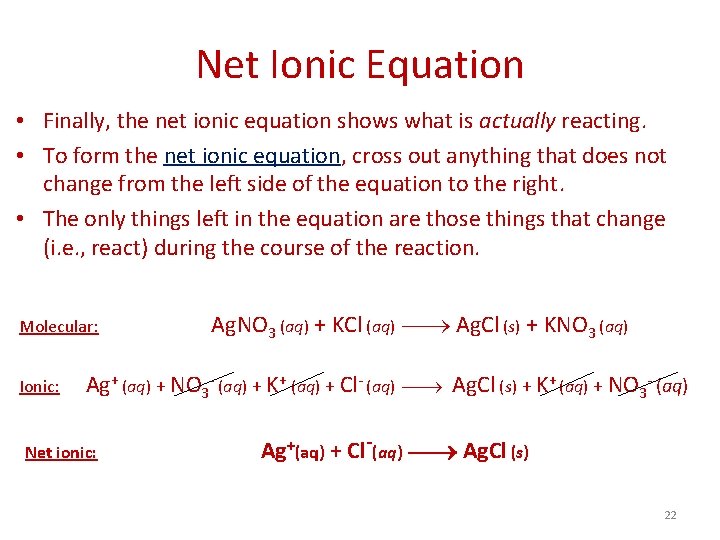
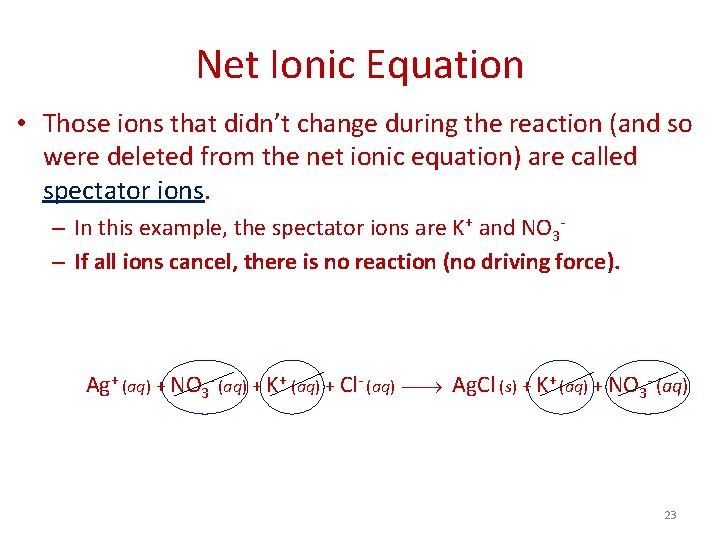
Net Ionic Equation • Finally, the net ionic equation shows what is actually reacting. • To form the net ionic equation, cross out anything that does not change from the left side of the equation to the right. • The only things left in the equation are those things that change (i. e. , react) during the course of the reaction. Molecular: Ionic: Ag. NO 3 (aq) + KCl (aq) Ag. Cl (s) + KNO 3 (aq) Ag+ (aq) + NO 3 - (aq) + K+ (aq) + Cl- (aq) Ag. Cl (s) + K+ (aq) + NO 3 - (aq) Net ionic: Ag+(aq) + Cl-(aq) Ag. Cl (s) 22

Net Ionic Equation • Those ions that didn’t change during the reaction (and so were deleted from the net ionic equation) are called spectator ions. – In this example, the spectator ions are K+ and NO 3– If all ions cancel, there is no reaction (no driving force). Ag+ (aq) + NO 3 - (aq) + K+ (aq) + Cl- (aq) Ag. Cl (s) + K+ (aq) + NO 3 - (aq) 23

Writing Net Ionic Equations 1. 2. 3. 4. Write a balanced molecular equation. Write the ionic equation by dissociating all soluble, strong electrolytes. (Remember to use coefficients to indicate how many of each ion were in the molecular equation. ) Cross out anything that remains unchanged from the left side to the right side of the equation (spectator ions). Write the net ionic equation with the species that remain. (Should already be balanced) 24

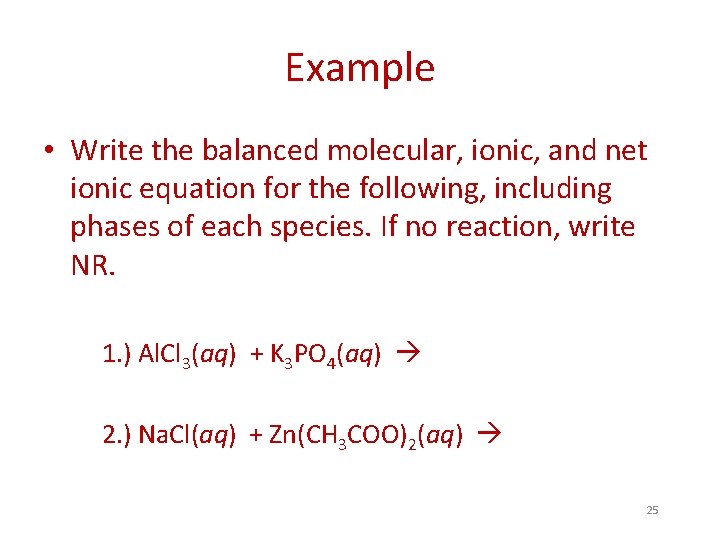
Example • Write the balanced molecular, ionic, and net ionic equation for the following, including phases of each species. If no reaction, write NR. 1. ) Al. Cl 3(aq) + K 3 PO 4(aq) 2. ) Na. Cl(aq) + Zn(CH 3 COO)2(aq) 25

Acids • Acids – substance that ionizes in aqueous solution to form hydrogen ion (H+), or a proton • So we say acids are proton donors – Monoprotic acids yield one H+ per molecule of acid • Ex: HNO 3, HCl(aq) H+(aq) + Cl-(aq) – Diprotic acids yield two H+ ions per molecule of acid • Ex: H 2 SO 4(aq) H+(aq) + HSO 4 -(aq) HSO 4 -(aq) H+(aq) + SO 42 -(aq) 26

Bases • Bases are substances that accept (react with) H+ ions • Bases produce hydroxide inos (OH-) when dissolved in water – Some include OH- in formula: • Na. OH, Ca(OH)2 – Some don’t include OH- in formula: • NH 3 (only one you should worry about for now) 27

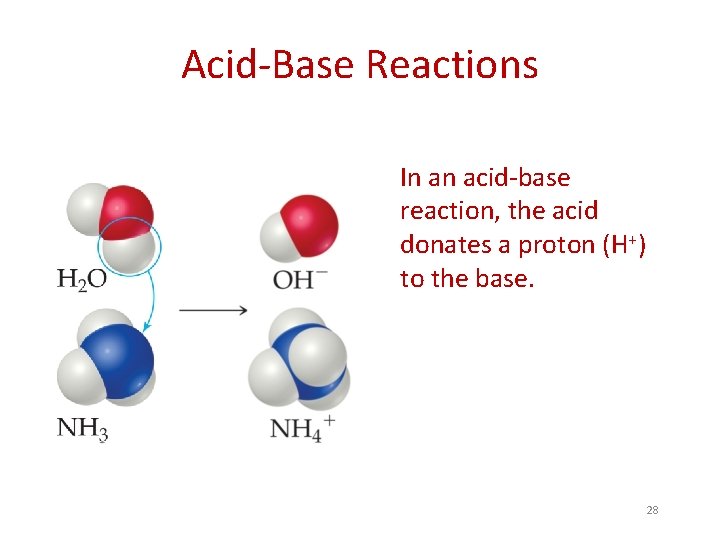
Acid-Base Reactions In an acid-base reaction, the acid donates a proton (H+) to the base. 28

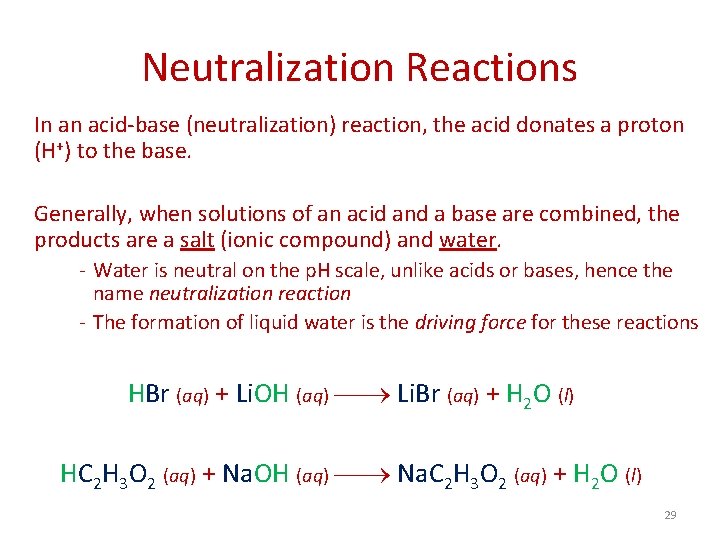
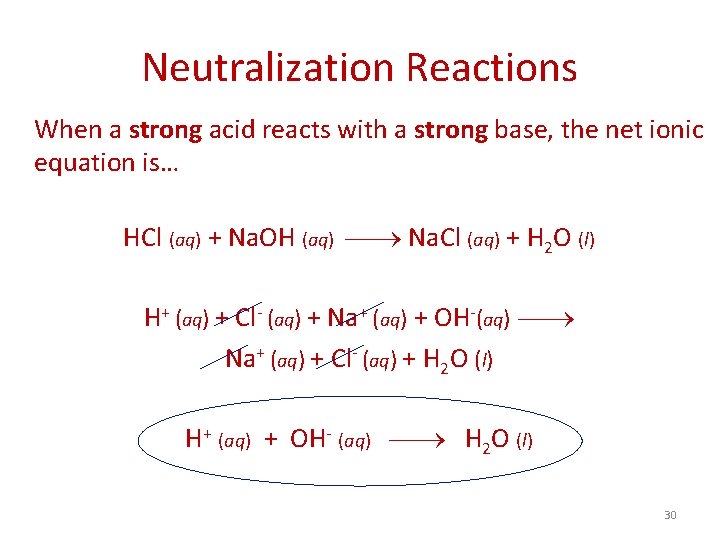
Neutralization Reactions In an acid-base (neutralization) reaction, the acid donates a proton (H+) to the base. Generally, when solutions of an acid and a base are combined, the products are a salt (ionic compound) and water. - Water is neutral on the p. H scale, unlike acids or bases, hence the name neutralization reaction - The formation of liquid water is the driving force for these reactions HBr (aq) + Li. OH (aq) Li. Br (aq) + H 2 O (l) HC 2 H 3 O 2 (aq) + Na. OH (aq) Na. C 2 H 3 O 2 (aq) + H 2 O (l) 29

Neutralization Reactions When a strong acid reacts with a strong base, the net ionic equation is… HCl (aq) + Na. OH (aq) Na. Cl (aq) + H 2 O (l) H+ (aq) + Cl- (aq) + Na+ (aq) + OH-(aq) Na+ (aq) + Cl- (aq) + H 2 O (l) H+ (aq) + OH- (aq) H 2 O (l) 30

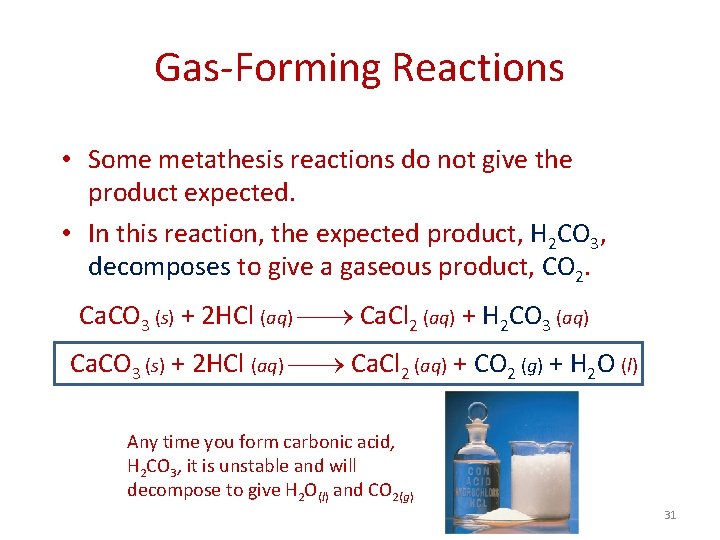
Gas-Forming Reactions • Some metathesis reactions do not give the product expected. • In this reaction, the expected product, H 2 CO 3, decomposes to give a gaseous product, CO 2. Ca. CO 3 (s) + 2 HCl (aq) Ca. Cl 2 (aq) + H 2 CO 3 (aq) Ca. CO 3 (s) + 2 HCl (aq) Ca. Cl 2 (aq) + CO 2 (g) + H 2 O (l) Any time you form carbonic acid, H 2 CO 3, it is unstable and will decompose to give H 2 O(l) and CO 2(g) 31

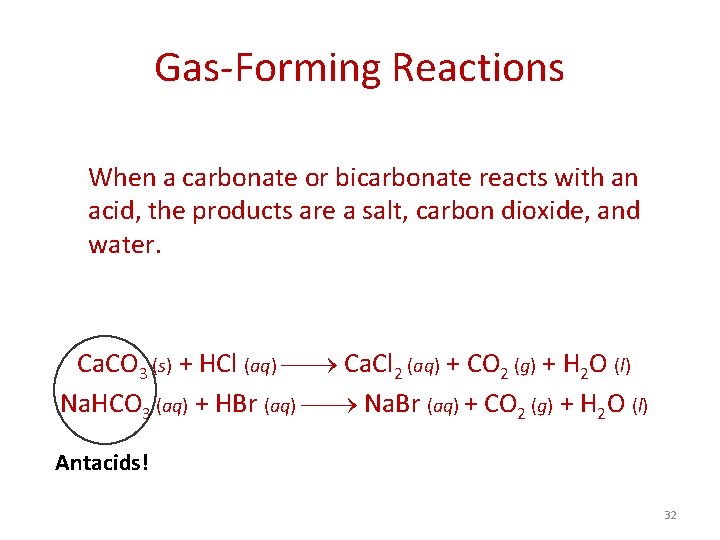
Gas-Forming Reactions When a carbonate or bicarbonate reacts with an acid, the products are a salt, carbon dioxide, and water. Ca. CO 3 (s) + HCl (aq) Ca. Cl 2 (aq) + CO 2 (g) + H 2 O (l) Na. HCO 3 (aq) + HBr (aq) Na. Br (aq) + CO 2 (g) + H 2 O (l) Antacids! 32

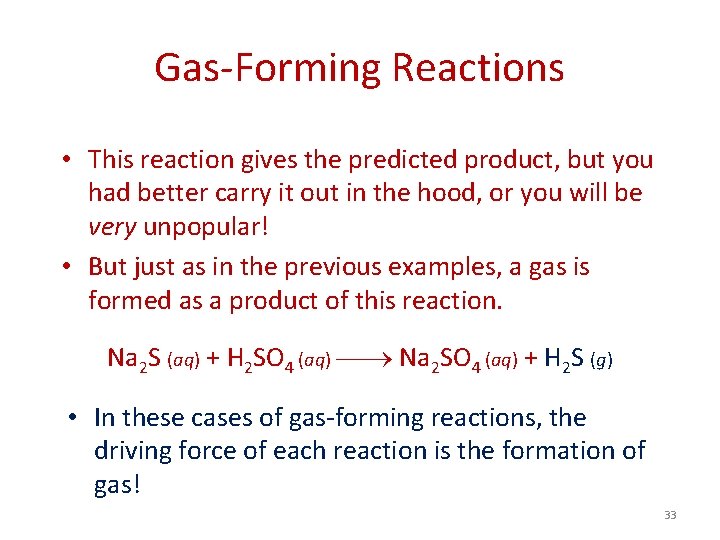
Gas-Forming Reactions • This reaction gives the predicted product, but you had better carry it out in the hood, or you will be very unpopular! • But just as in the previous examples, a gas is formed as a product of this reaction. Na 2 S (aq) + H 2 SO 4 (aq) Na 2 SO 4 (aq) + H 2 S (g) • In these cases of gas-forming reactions, the driving force of each reaction is the formation of gas! 33

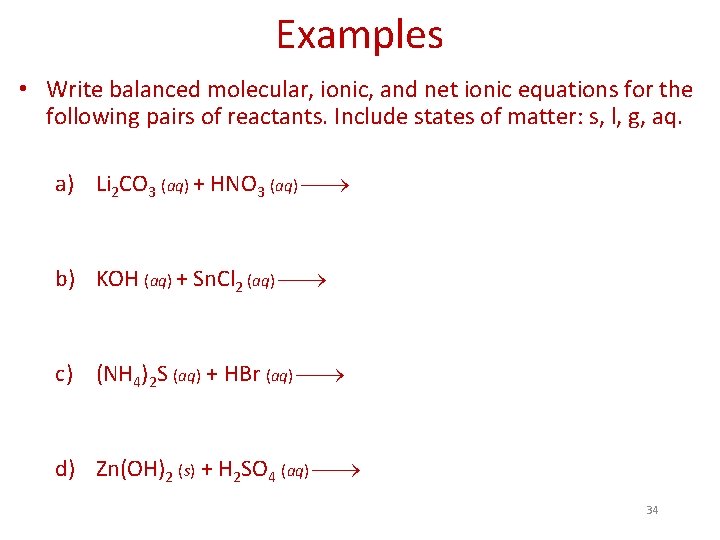
Examples • Write balanced molecular, ionic, and net ionic equations for the following pairs of reactants. Include states of matter: s, l, g, aq. a) Li 2 CO 3 (aq) + HNO 3 (aq) b) KOH (aq) + Sn. Cl 2 (aq) c) (NH 4)2 S (aq) + HBr (aq) d) Zn(OH)2 (s) + H 2 SO 4 (aq) 34

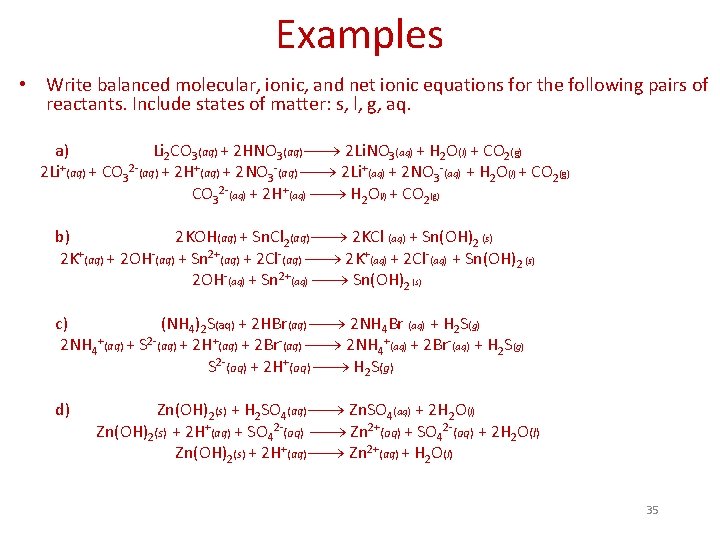
Examples • Write balanced molecular, ionic, and net ionic equations for the following pairs of reactants. Include states of matter: s, l, g, aq. a) Li 2 CO 3(aq) + 2 HNO 3(aq) 2 Li. NO 3(aq) + H 2 O(l) + CO 2(g) 2 Li+(aq) + CO 32 -(aq) + 2 H+(aq) + 2 NO 3 -(aq) 2 Li+(aq) + 2 NO 3 -(aq) + H 2 O(l) + CO 2(g) CO 32 -(aq) + 2 H+(aq) H 2 O(l) + CO 2(g) b) 2 KOH(aq) + Sn. Cl 2(aq) 2 KCl (aq) + Sn(OH)2 (s) 2 K+(aq) + 2 OH-(aq) + Sn 2+(aq) + 2 Cl-(aq) 2 K+(aq) + 2 Cl-(aq) + Sn(OH)2 (s) 2 OH-(aq) + Sn 2+(aq) Sn(OH)2 (s) c) (NH 4)2 S(aq) + 2 HBr(aq) 2 NH 4 Br (aq) + H 2 S(g) 2 NH 4+(aq) + S 2 -(aq) + 2 H+(aq) + 2 Br-(aq) 2 NH 4+(aq) + 2 Br-(aq) + H 2 S(g) S 2 -(aq) + 2 H+(aq) H 2 S(g) d) Zn(OH)2(s) + H 2 SO 4(aq) Zn. SO 4(aq) + 2 H 2 O(l) Zn(OH)2(s) + 2 H+(aq) + SO 42 -(aq) Zn 2+(aq) + SO 42 -(aq) + 2 H 2 O(l) Zn(OH)2(s) + 2 H+(aq) Zn 2+(aq) + H 2 O(l) 35

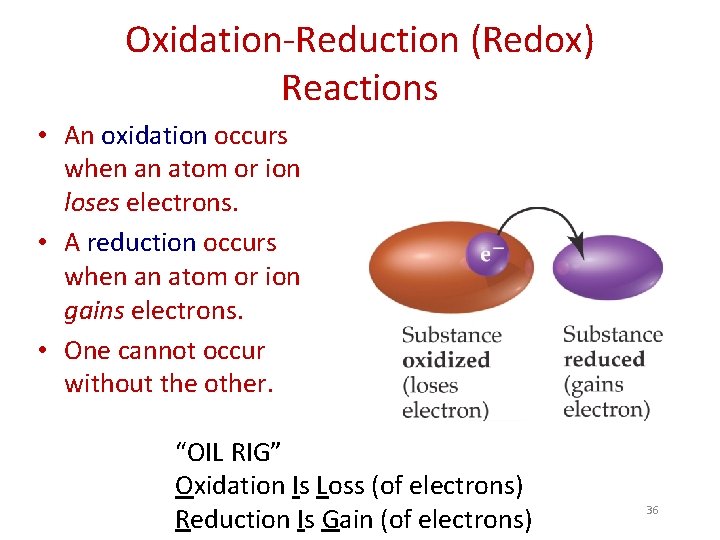
Oxidation-Reduction (Redox) Reactions • An oxidation occurs when an atom or ion loses electrons. • A reduction occurs when an atom or ion gains electrons. • One cannot occur without the other. “OIL RIG” Oxidation Is Loss (of electrons) Reduction Is Gain (of electrons) 36

Oxidation Numbers Oxidation numbers are for bookkeeping of electrons. To determine if an oxidation-reduction reaction has occurred, we assign an oxidation number (or oxidation state) to each element in a neutral compound or charged entity. 37

Rules for Assigning Oxidation Numbers 1. Elements in their elemental form have an oxidation number of 0. • Examples: Na, O 2, H 2, Cu, Ag, etc… all have oxidation numbers of 0 Note: the remaining rules are for elements when they are part of a compound 2. The oxidation number of a monatomic ion is the same as its charge. • Examples: Na+ Cl. O 2 Cu 2+ oxidation number = +1 oxidation number = -2 oxdiation number = +2 38

Rules for Assigning Oxidation Numbers 3. Oxygen has an oxidation number of − 2, except in the peroxide ion O 22 - in which it has an oxidation number of − 1. 4. Hydrogen is − 1 when bonded to a metal, +1 when bonded to a nonmetal. 5. Fluorine always has an oxidation number of − 1. • The other halogens have an oxidation number of − 1 when they are monatomic ions; they can have positive oxidation numbers, however, most notably in oxyanions. 39

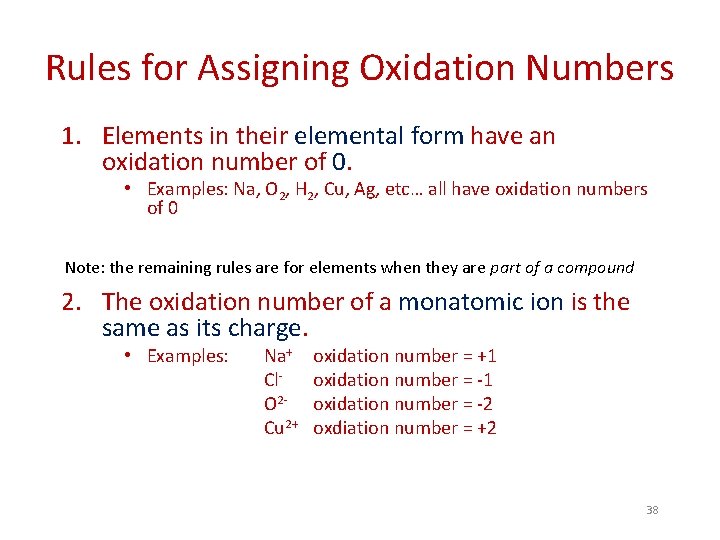
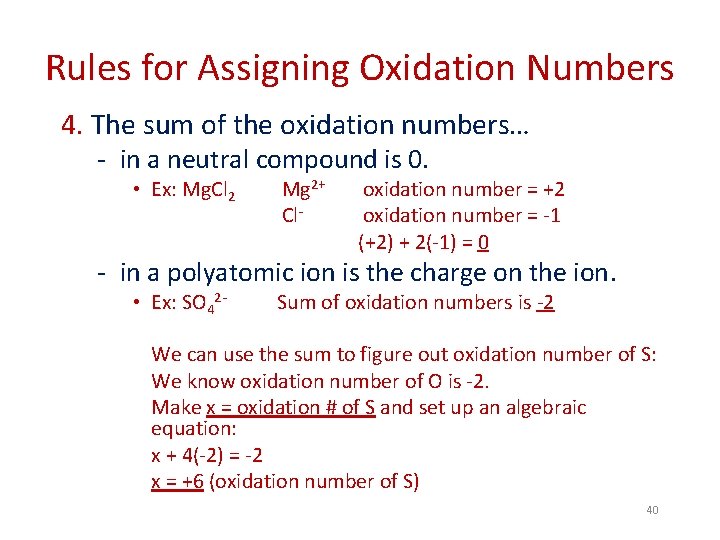
Rules for Assigning Oxidation Numbers 4. The sum of the oxidation numbers… - in a neutral compound is 0. • Ex: Mg. Cl 2 Mg 2+ Cl- oxidation number = +2 oxidation number = -1 (+2) + 2(-1) = 0 - in a polyatomic ion is the charge on the ion. • Ex: SO 42 - Sum of oxidation numbers is -2 We can use the sum to figure out oxidation number of S: We know oxidation number of O is -2. Make x = oxidation # of S and set up an algebraic equation: x + 4(-2) = -2 x = +6 (oxidation number of S) 40

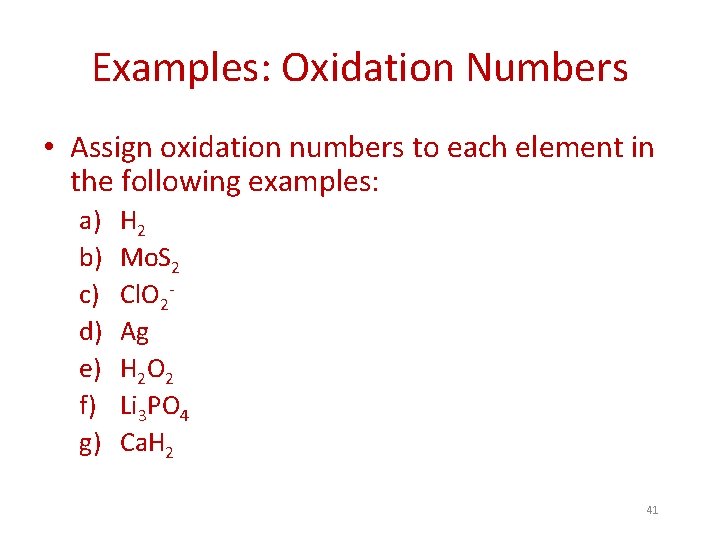
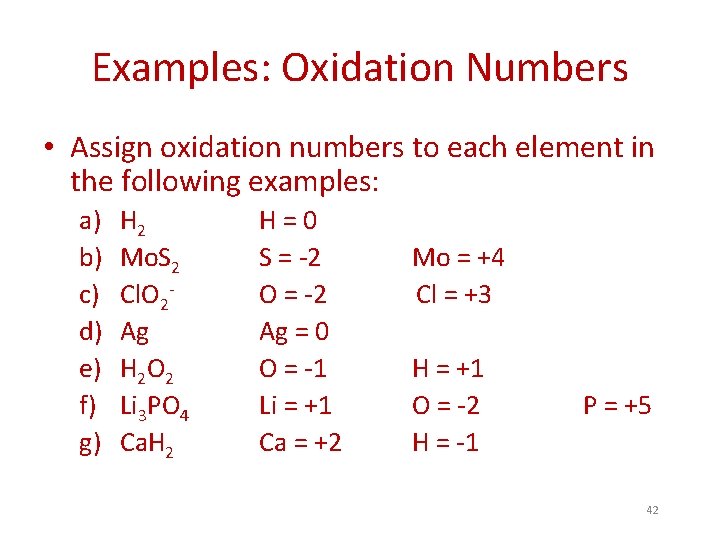
Examples: Oxidation Numbers • Assign oxidation numbers to each element in the following examples: a) b) c) d) e) f) g) H 2 Mo. S 2 Cl. O 2 Ag H 2 O 2 Li 3 PO 4 Ca. H 2 41

Examples: Oxidation Numbers • Assign oxidation numbers to each element in the following examples: a) b) c) d) e) f) g) H 2 Mo. S 2 Cl. O 2 Ag H 2 O 2 Li 3 PO 4 Ca. H 2 H=0 S = -2 O = -2 Ag = 0 O = -1 Li = +1 Ca = +2 Mo = +4 Cl = +3 H = +1 O = -2 H = -1 P = +5 42

Displacement Reactions A + BX AX + B • In displacement reactions, ion B oxidizes an element A. • The ion B, then, is reduced to elemental form. Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2(g) 43

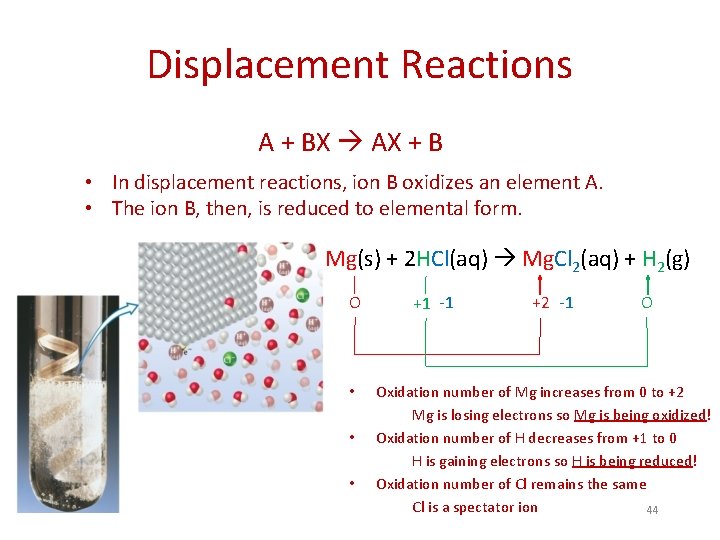
Displacement Reactions A + BX AX + B • In displacement reactions, ion B oxidizes an element A. • The ion B, then, is reduced to elemental form. Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2(g) O • • • +1 -1 +2 -1 O Oxidation number of Mg increases from 0 to +2 Mg is losing electrons so Mg is being oxidized! Oxidation number of H decreases from +1 to 0 H is gaining electrons so H is being reduced! Oxidation number of Cl remains the same Cl is a spectator ion 44

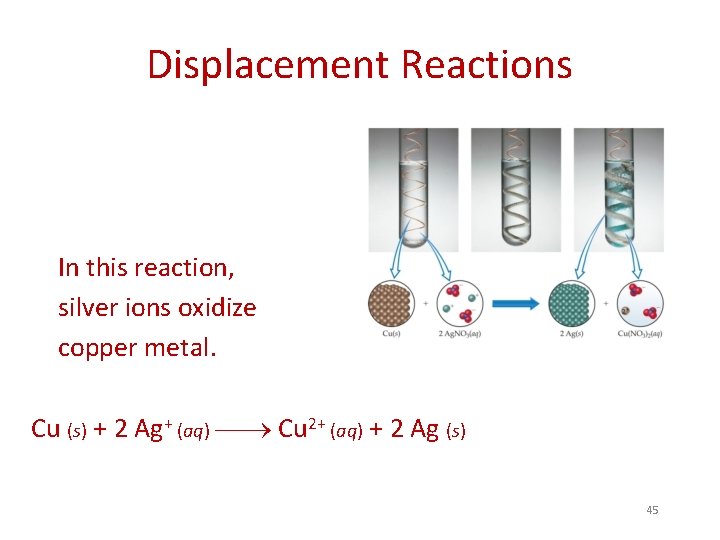
Displacement Reactions In this reaction, silver ions oxidize copper metal. Cu (s) + 2 Ag+ (aq) Cu 2+ (aq) + 2 Ag (s) 45

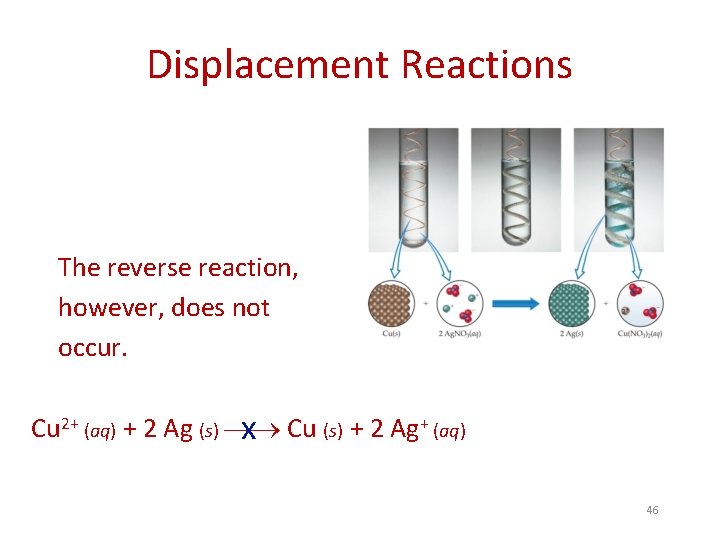
Displacement Reactions The reverse reaction, however, does not occur. Cu 2+ (aq) + 2 Ag (s) x Cu (s) + 2 Ag+ (aq) 46

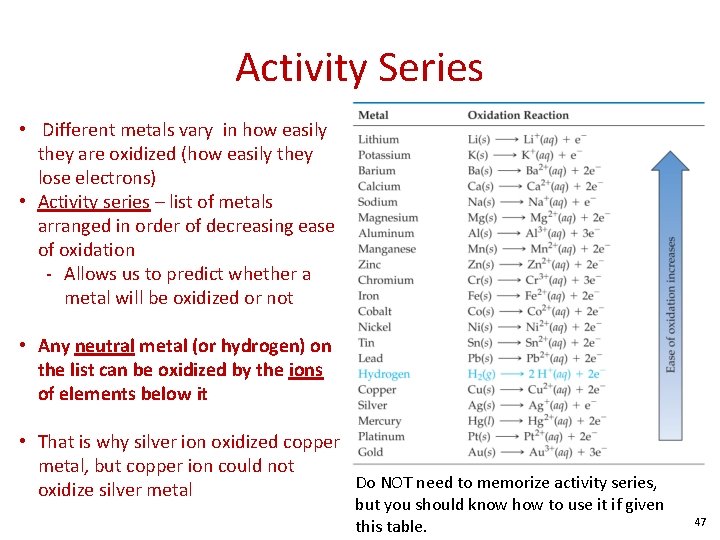
Activity Series • Different metals vary in how easily they are oxidized (how easily they lose electrons) • Activity series – list of metals arranged in order of decreasing ease of oxidation - Allows us to predict whether a metal will be oxidized or not • Any neutral metal (or hydrogen) on the list can be oxidized by the ions of elements below it • That is why silver ion oxidized copper metal, but copper ion could not Do NOT need to memorize activity series, oxidize silver metal but you should know how to use it if given this table. 47

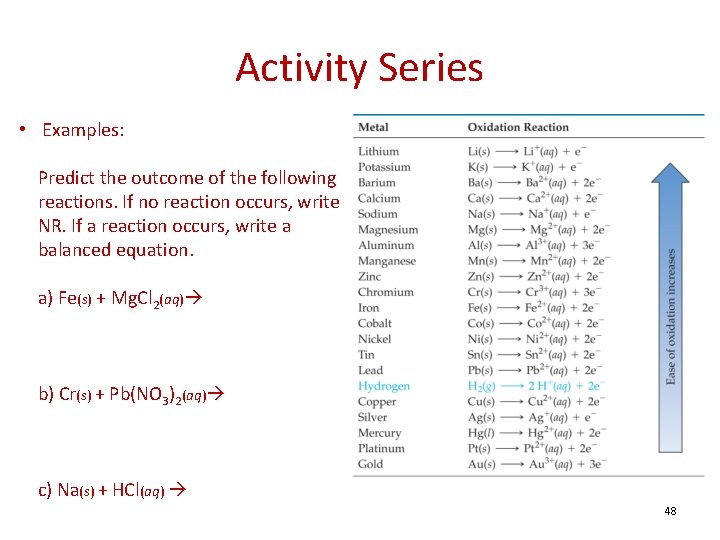
Activity Series • Examples: Predict the outcome of the following reactions. If no reaction occurs, write NR. If a reaction occurs, write a balanced equation. a) Fe(s) + Mg. Cl 2(aq) b) Cr(s) + Pb(NO 3)2(aq) c) Na(s) + HCl(aq) 48

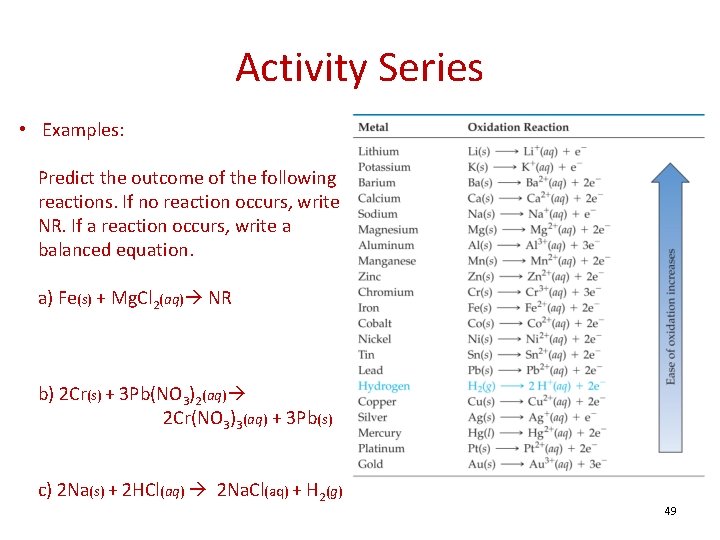
Activity Series • Examples: Predict the outcome of the following reactions. If no reaction occurs, write NR. If a reaction occurs, write a balanced equation. a) Fe(s) + Mg. Cl 2(aq) NR b) 2 Cr(s) + 3 Pb(NO 3)2(aq) 2 Cr(NO 3)3(aq) + 3 Pb(s) c) 2 Na(s) + 2 HCl(aq) 2 Na. Cl(aq) + H 2(g) 49

Molarity • Concentration – tells how much solute is dissolved in a solvent • Molarity is one way to measure the concentration of a solution. moles of solute Molarity (M) = volume of solution in liters Ex: 2. 1 M Mg. Cl 2 = 2. 1 mol Mg. Cl 2/L Mg. Cl 2 solution (read as: ) “ 2. 1 molar solution of Mg. Cl 2” • Because the units of molarity are mol/L, we can use molarity as a conversion factor in stoichiometric calculations to interconvert: moles of solute liters of solution 2. 1 mol Mg. Cl 2 1 L solution or 1 L solution 2. 1 mol Mg. Cl 2 50

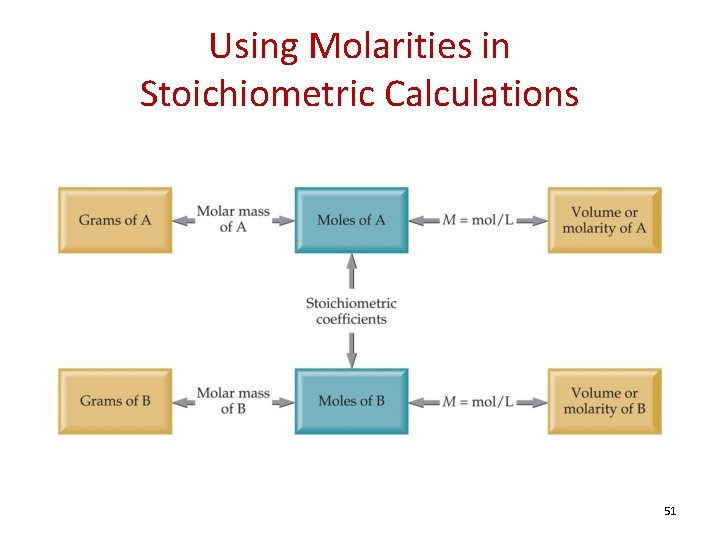
Using Molarities in Stoichiometric Calculations 51

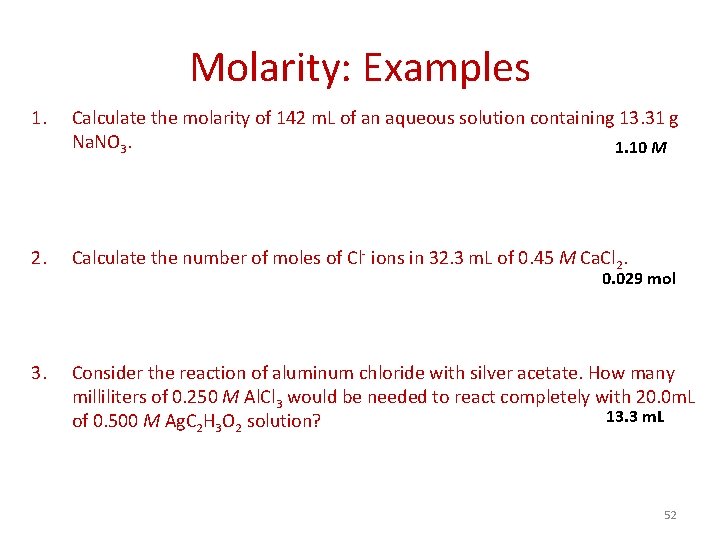
Molarity: Examples 1. Calculate the molarity of 142 m. L of an aqueous solution containing 13. 31 g Na. NO 3. 1. 10 M 2. Calculate the number of moles of Cl- ions in 32. 3 m. L of 0. 45 M Ca. Cl 2. 3. Consider the reaction of aluminum chloride with silver acetate. How many milliliters of 0. 250 M Al. Cl 3 would be needed to react completely with 20. 0 m. L 13. 3 m. L of 0. 500 M Ag. C 2 H 3 O 2 solution? 0. 029 mol 52

Dilution: Adding water If an original solution is diluted, the molarity of the new solution can be determined from the equation: M 1 V 1 = M 2 V 2 moles of solute before dilution = moles of solute after dilution (because all we are doing is adding solvent to the original solution) where M 1 and M 2 are the molarity of the initial (concentrated) and final (dilute) solutions, respectively, and V 1 and V 2 are the volumes of the two solutions. 53

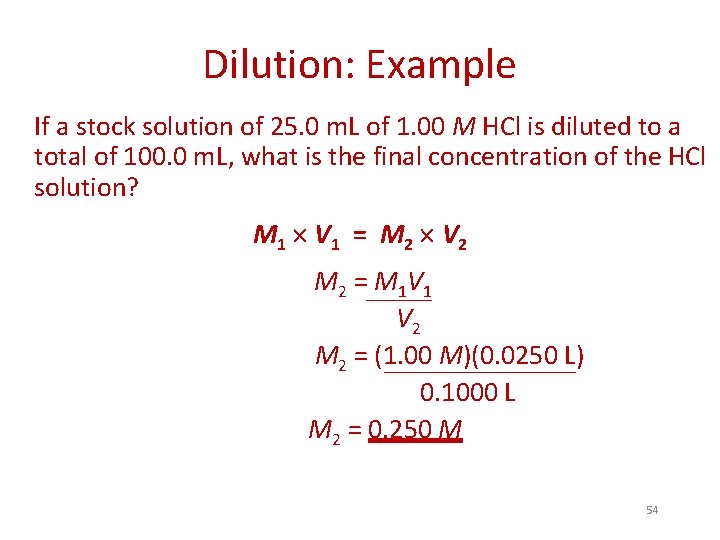
Dilution: Example If a stock solution of 25. 0 m. L of 1. 00 M HCl is diluted to a total of 100. 0 m. L, what is the final concentration of the HCl solution? M 1 V 1 = M 2 V 2 M 2 = M 1 V 2 M 2 = (1. 00 M)(0. 0250 L) 0. 1000 L M 2 = 0. 250 M 54

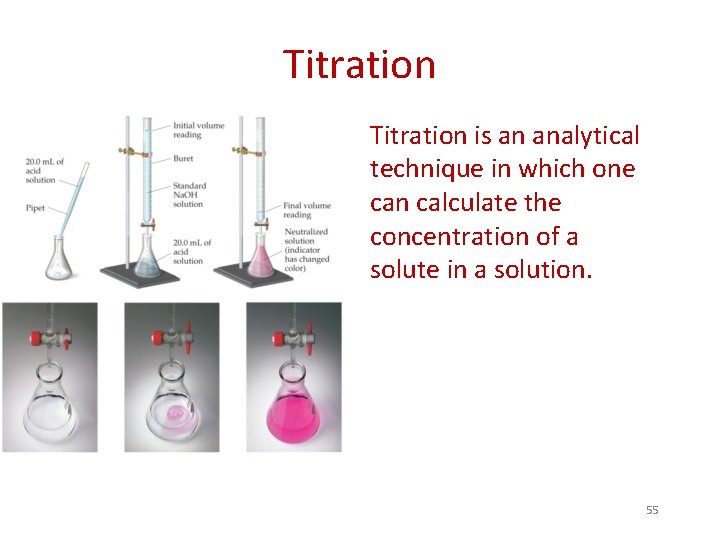
Titration is an analytical technique in which one can calculate the concentration of a solute in a solution. 55

Titration • A sample of known concentration (standard solution) is added to solution of unknown concentration. • The point at which stoichiometrically equivalent quantities are reached is the equivalence point. • For acid-base reactions, the equivalence point is observed with an indicator. – Ex: Phenolphthalein is colorless in acidic solutions but turns pink in basic solutions. 56

Titration Example In a titration, 23. 25 m. L of 0. 105 M Na. OH was needed to react with 21. 45 m. L of H 2 SO 4 solution. What is the molarity of the acid? 0. 0569 M 57