14 Metric facts 1 meter was originally defined

- Slides: 2

14. Metric facts: 1 meter was originally defined as the distance from equator to north pole divided by? 10 million One tenth of a meter is called a decimeter How many centimeters (cm) in one meter? 100 How many millimeters (mm) in one meter? 1000 The volume of 1, 000 cubic centimeters (cm 3 or cc) is called a liter. How many milliliters (m. L) are in a liter? 1000 A milliliter (m. L) is what fraction of a liter? 1 thousandth A milliliter is how many cubic centimeters? 1 A cubic centimeter of water has the mass of how many grams? 1 A liter of water has the mass of how many grams? 1000 15. It’s December and you want to heat up your pool. It is currently 50 o. F (10 o. C) and you want it raised to 80 o. F (27 o. C) Using an electric heater, how much will it cost if 1 kilowatt-hour is 11 cents? The pool is 7. 0 meters long, 5. 0 meters wide and 2. 0 7. 0 m x 5. 0 m x 2. 0 m= 70. meters deep. m 3 70 m x 100 cm/mx 100 cm/m= A. What’s the volume in cubic meters? ______ 3 3 70, 000 cm since 1 cm 3 water=1 g 70, 000 B. What is the volume in cubic grams centimeters? ______ A calorie is defined as the energy needed to raise one gram of water one degree C. What is the total grams of the water? Celsius. o. C to 27 o. C? o D. How many calories will it take to raise the pool water from 10 70, 000 grams 17 C 1 calorie = 1, 190, 000 calories or ______ o g. C 1. 19 x 109 calories ________ 1. 19 x 109 calories 1. 163 x 10 -6 kilowatt-hrs = 1. 38 x 103 kilowatt-hours -6 kilowatts hours. How many kilowatt-hours will you need? E. 1 calorie = 1. 163 x 10 calori 1. 384 x 103 kilowatt-hours $0. 11 = es kilowatt-hr ______ $152. 24 16. For temperatures we see in Phoenix, a quick way to do an approximate conversion in your head for Fahrenheit to Celsius is to subtract 30, add 5, and then o. F (10 o. C) to 80 o. F (27 o. C)? F. How much will it cost to heat the pool from 50 cut in half. For Celsius to Fahrenheit, double then add 25. Use this method for the following conversions. Exact is to subtract 32 and multiply by 1. 8 ________ 52 o. F to o. C: 13 or 14 o. C 37 o. C to o. F: 99 o. F 101 o. F to o. C: 38 o. C 25 o. C to o. F: 75 o. F 70 o. F to o. C: 22 or 23 o. C 10 o. C to o. F: 45 o. F 125 o. F to o. C: 50 o. C 80 o. C to o. F: 185 o. F

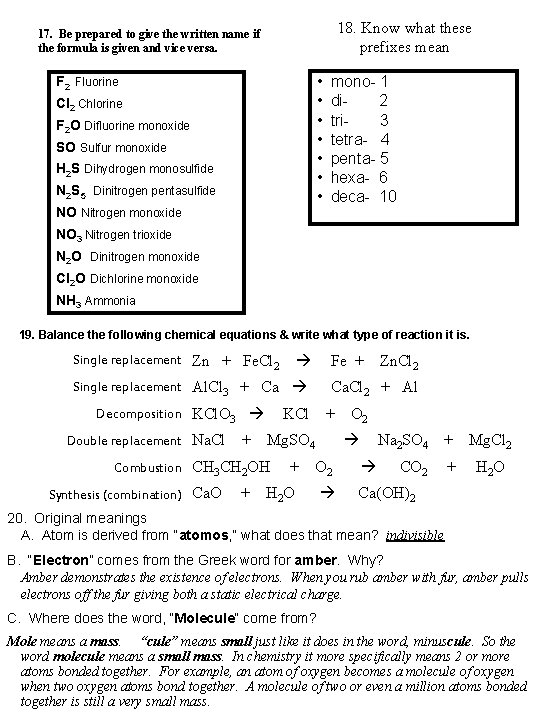
18. Know what these prefixes mean 17. Be prepared to give the written name if the formula is given and vice versa. • • F 2 Fluorine Cl 2 Chlorine F 2 O Difluorine monoxide SO Sulfur monoxide H 2 S Dihydrogen monosulfide N 2 S 5 Dinitrogen pentasulfide NO Nitrogen monoxide mono- 1 di- 2 tri- 3 tetra- 4 penta- 5 hexa- 6 deca- 10 NO 3 Nitrogen trioxide N 2 O Dinitrogen monoxide Cl 2 O Dichlorine monoxide NH 3 Ammonia 19. Balance the following chemical equations & write what type of reaction it is. Single replacement Zn + Fe. Cl 2 Fe + Single replacement Al. Cl 3 + Ca Decomposition KCl. O 3 Double replacement Na. Cl + + Ca. Cl 2 + Al KCl + O 2 Mg. SO 4 Na 2 SO 4 + Mg. Cl 2 CO 2 H 2 O + O 2 H 2 O Ca(OH)2 Combustion CH 3 CH 2 OH Synthesis (combination) Ca. O Zn. Cl 2 + 20. Original meanings A. Atom is derived from “atomos, ” what does that mean? indivisible B. “Electron” comes from the Greek word for amber. Why? Amber demonstrates the existence of electrons. When you rub amber with fur, amber pulls electrons off the fur giving both a static electrical charge. C. Where does the word, “Molecule” come from? Mole means a mass. “cule” means small just like it does in the word, minuscule. So the word molecule means a small mass. In chemistry it more specifically means 2 or more atoms bonded together. For example, an atom of oxygen becomes a molecule of oxygen when two oxygen atoms bond together. A molecule of two or even a million atoms bonded together is still a very small mass.