14 8 Theoretical Models for Chemical Kinetics Collision



- Slides: 29

14 -8 Theoretical Models for Chemical Kinetics Collision Theory ¨ Kinetic-Molecular theory can be used to calculate the collision frequency. · In gases 1030 collisions per second. · If each collision produced a reaction, the rate would be about 106 M s-1. · Actual rates are on the order of 104 M s-1. ◦ Still a very rapid rate. · Only a fraction of collisions yield a reaction. 1 General Chemistry: Chapter 14 Prentice-Hall © 2007

Activation Energy ¨ For a reaction to occur there must be a redistribution of energy sufficient to break certain bonds in the reacting molecule(s). ¨ Activation Energy: · The minimum energy above the average kinetic energy that molecules must bring to their collisions for a chemical reaction to occur. 2 General Chemistry: Chapter 14 Prentice-Hall © 2007

Activation Energy 3 General Chemistry: Chapter 14 Prentice-Hall © 2007

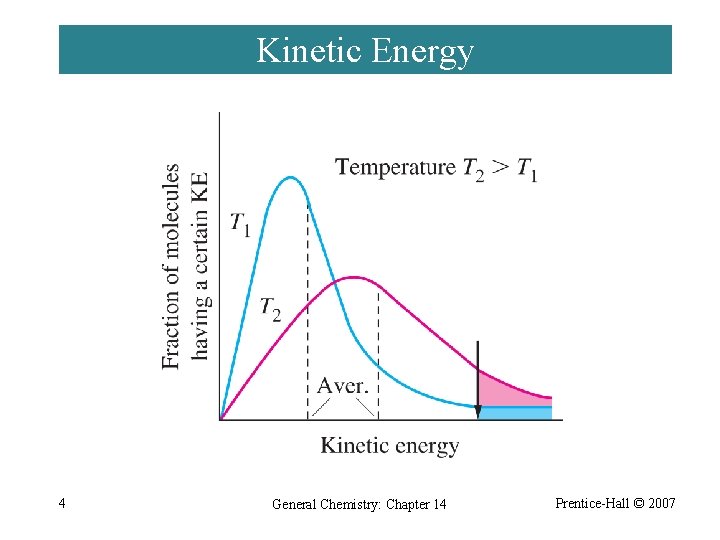
Kinetic Energy 4 General Chemistry: Chapter 14 Prentice-Hall © 2007

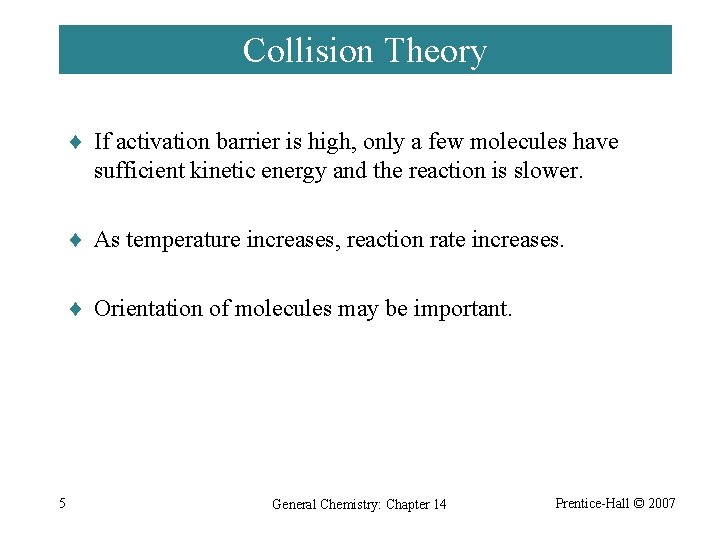
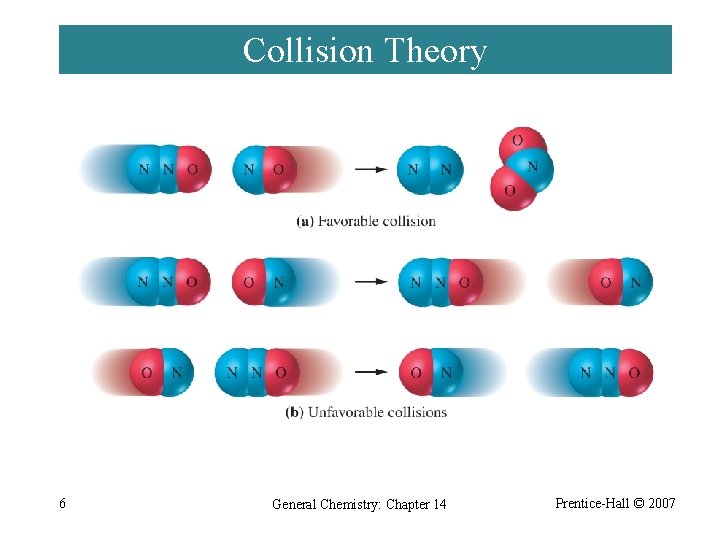
Collision Theory ¨ If activation barrier is high, only a few molecules have sufficient kinetic energy and the reaction is slower. ¨ As temperature increases, reaction rate increases. ¨ Orientation of molecules may be important. 5 General Chemistry: Chapter 14 Prentice-Hall © 2007

Collision Theory 6 General Chemistry: Chapter 14 Prentice-Hall © 2007

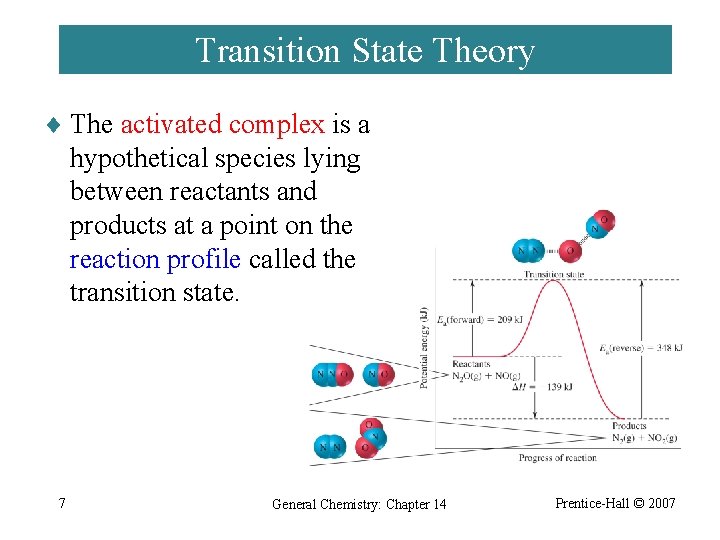
Transition State Theory ¨ The activated complex is a hypothetical species lying between reactants and products at a point on the reaction profile called the transition state. 7 General Chemistry: Chapter 14 Prentice-Hall © 2007

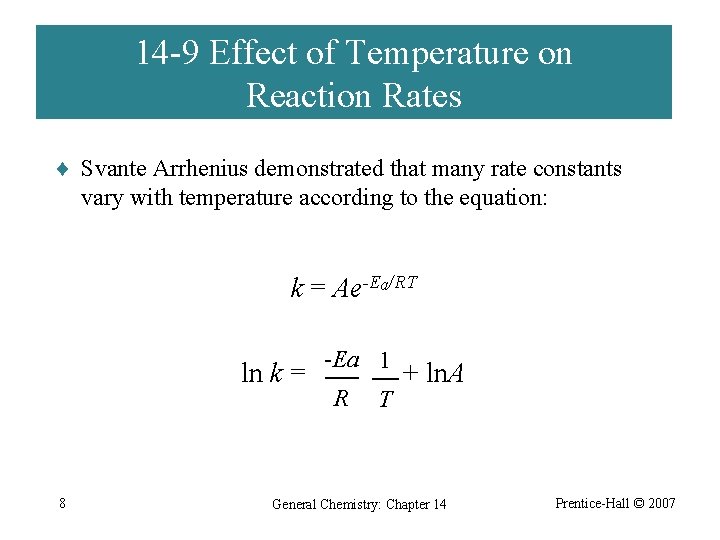
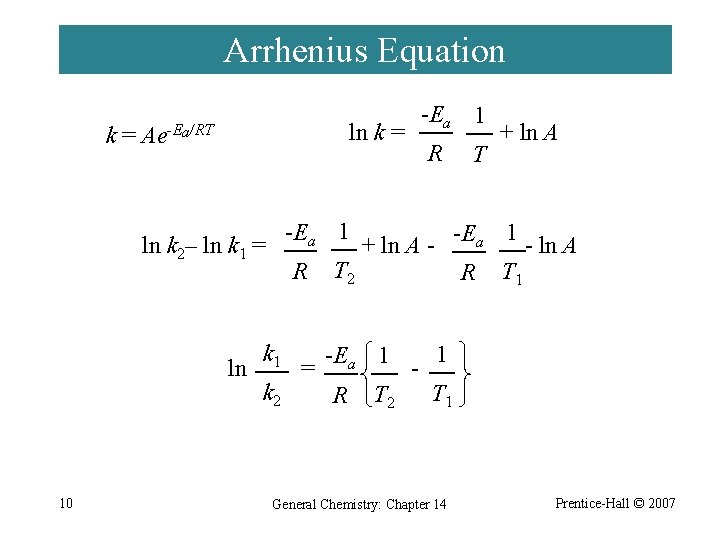
14 -9 Effect of Temperature on Reaction Rates ¨ Svante Arrhenius demonstrated that many rate constants vary with temperature according to the equation: k = Ae-Ea/RT ln k = 8 -Ea 1 R T + ln. A General Chemistry: Chapter 14 Prentice-Hall © 2007

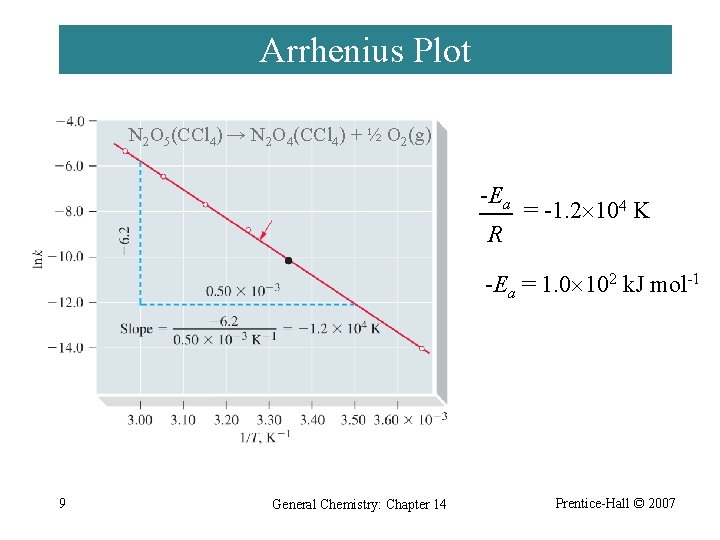
Arrhenius Plot N 2 O 5(CCl 4) → N 2 O 4(CCl 4) + ½ O 2(g) -Ea R = -1. 2 104 K -Ea = 1. 0 102 k. J mol-1 9 General Chemistry: Chapter 14 Prentice-Hall © 2007

Arrhenius Equation k= ln R T + ln A -Ea 1 ln k 2– ln k 1 = 10 -Ea 1 ln k = Ae-Ea/RT R k 1 k 2 = 1 -E a + ln A - ln A T 2 R T 1 -Ea 1 R T 2 - 1 T 1 General Chemistry: Chapter 14 Prentice-Hall © 2007

14 -10 Reaction Mechanisms ¨ A step-by-step description of a chemical reaction. ¨ Each step is called an elementary process. · Any molecular event that significantly alters a molecules energy of geometry or produces a new molecule. ¨ Reaction mechanism must be consistent with: · Stoichiometry for the overall reaction. · The experimentally determined rate law. 11 General Chemistry: Chapter 14 Prentice-Hall © 2007

Elementary Processes ¨ Unimolecular or bimolecular. ¨ Exponents for concentration terms are the same as the stoichiometric factors for the elementary process. ¨ Elementary processes are reversible. ¨ Intermediates are produced in one elementary process and consumed in another. ¨ One elementary step is usually slower than all the others and is known as the rate determining step. 12 General Chemistry: Chapter 14 Prentice-Hall © 2007

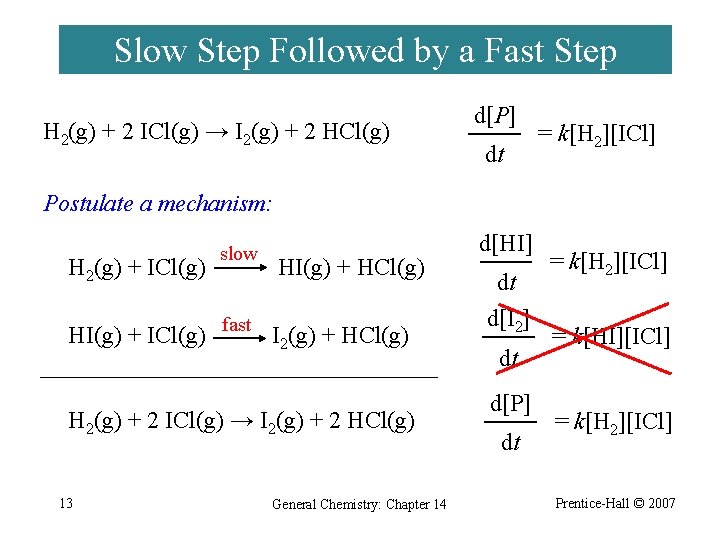
Slow Step Followed by a Fast Step H 2(g) + 2 ICl(g) → I 2(g) + 2 HCl(g) d[P] dt = k[H 2][ICl] Postulate a mechanism: H 2(g) + ICl(g) slow HI(g) + HCl(g) HI(g) + ICl(g) fast I 2(g) + HCl(g) H 2(g) + 2 ICl(g) → I 2(g) + 2 HCl(g) 13 General Chemistry: Chapter 14 d[HI] dt d[I 2] dt d[P] dt = k[H 2][ICl] = k[HI][ICl] = k[H 2][ICl] Prentice-Hall © 2007

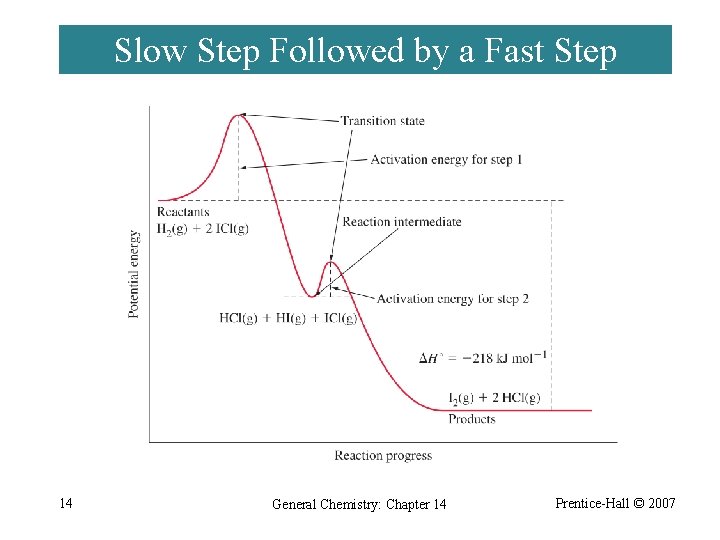
Slow Step Followed by a Fast Step 14 General Chemistry: Chapter 14 Prentice-Hall © 2007

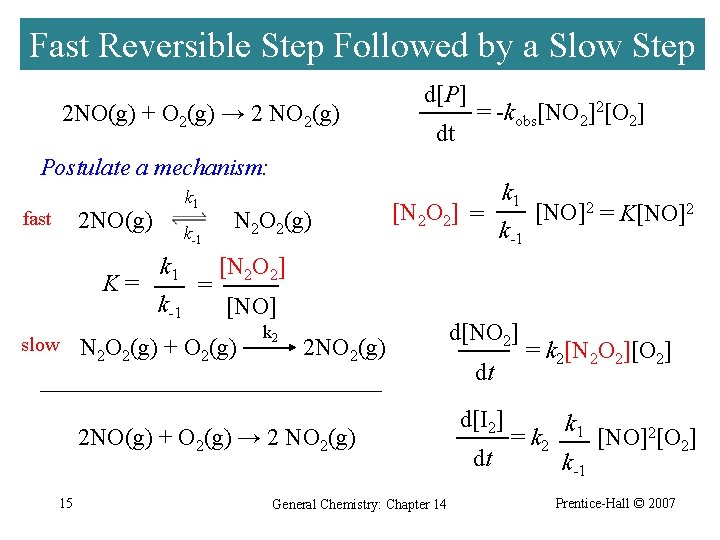
Fast Reversible Step Followed by a Slow Step 2 NO(g) + O 2(g) → 2 NO 2(g) d[P] dt = -kobs[NO 2]2[O 2] Postulate a mechanism: k 1 2 NO(g) fast K= slow k-1 k 1 k-1 = N 2 O 2(g) [N 2 O 2] = k-1 [NO]2 = K[NO]2 [N 2 O 2] [NO] N 2 O 2(g) + O 2(g) k 2 2 NO 2(g) 2 NO(g) + O 2(g) → 2 NO 2(g) 15 k 1 General Chemistry: Chapter 14 d[NO 2] dt d[I 2] dt = k 2[N 2 O 2][O 2] = k 2 k 1 k-1 [NO]2[O 2] Prentice-Hall © 2007

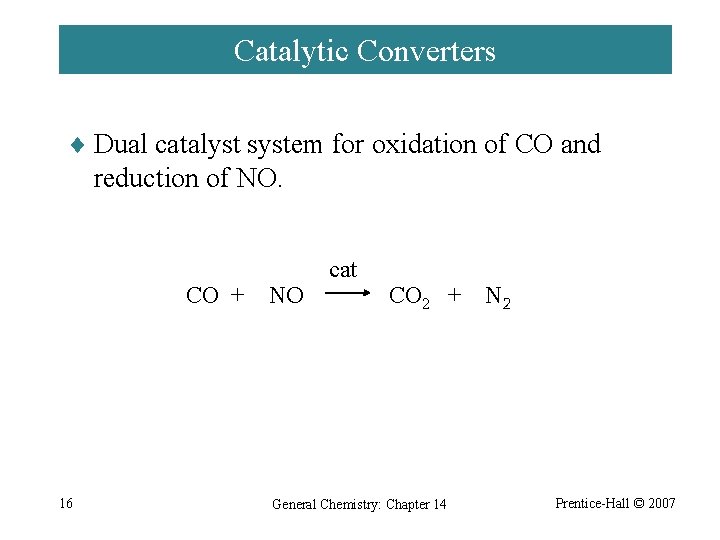
Catalytic Converters ¨ Dual catalyst system for oxidation of CO and reduction of NO. CO + 16 NO cat CO 2 + General Chemistry: Chapter 14 N 2 Prentice-Hall © 2007

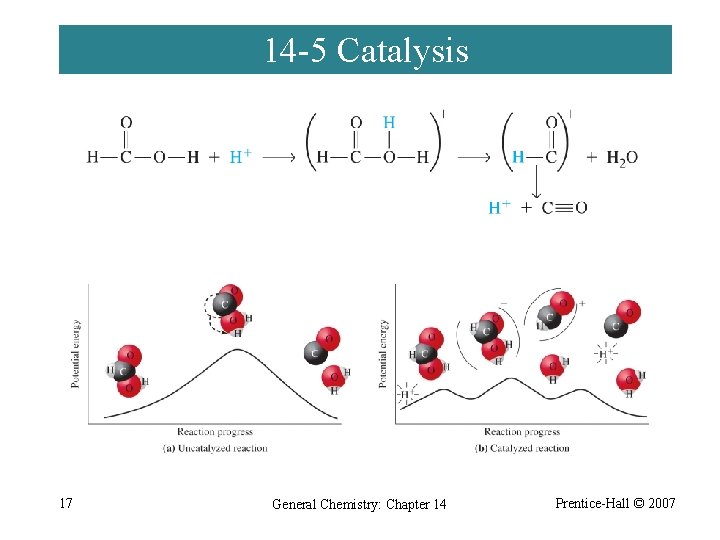
14 -5 Catalysis 17 General Chemistry: Chapter 14 Prentice-Hall © 2007

¨ Worked Examples Follow: 18 General Chemistry: Chapter 14 Prentice-Hall © 2007

19 General Chemistry: Chapter 14 Prentice-Hall © 2007

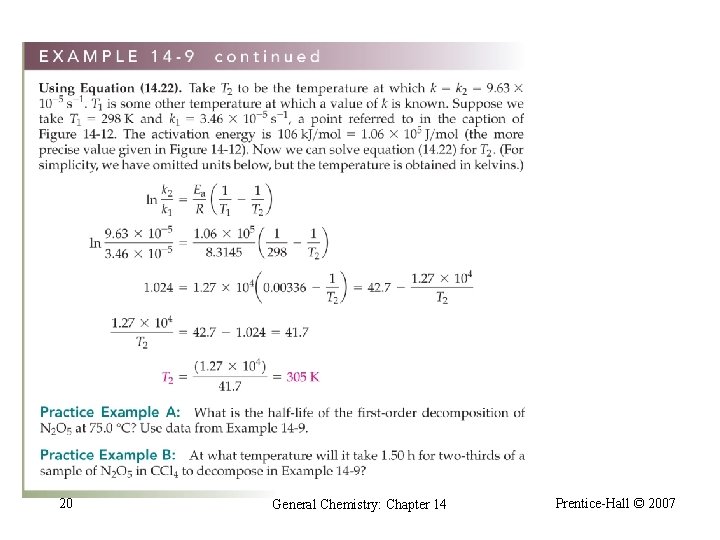
20 General Chemistry: Chapter 14 Prentice-Hall © 2007

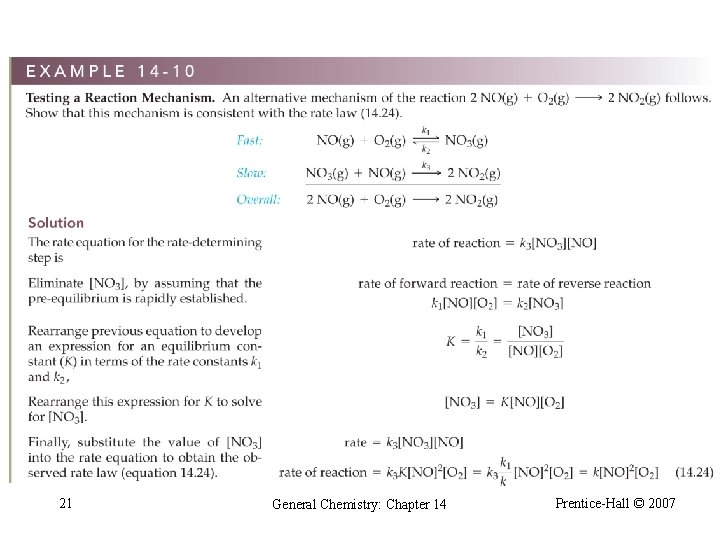
21 General Chemistry: Chapter 14 Prentice-Hall © 2007

¨ CRS Questions Follow: 22 General Chemistry: Chapter 14 Prentice-Hall © 2007

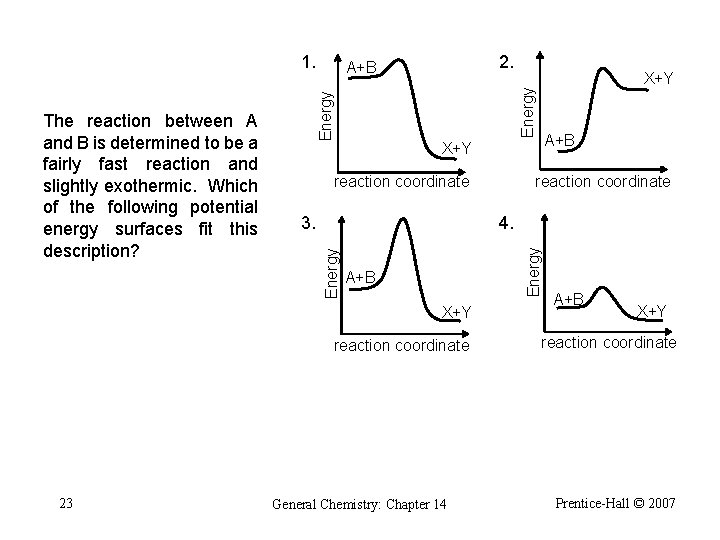
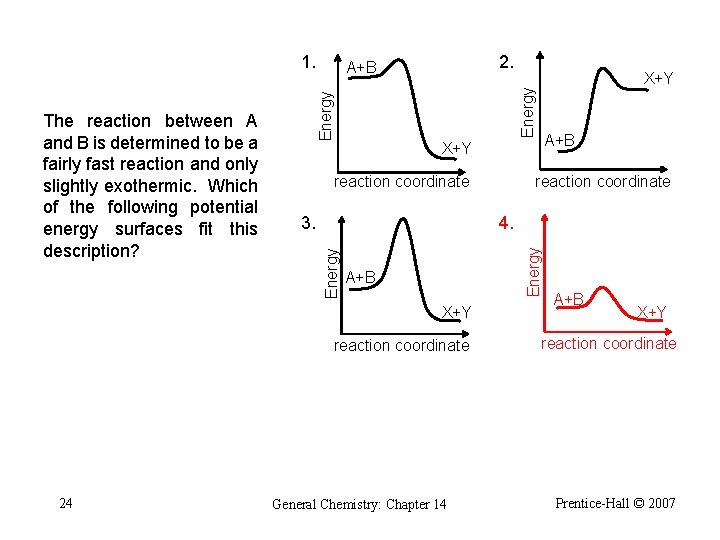
1. Energy A+B reaction coordinate 4. Energy 3. A+B X+Y reaction coordinate 23 X+Y reaction coordinate Energy The reaction between A and B is determined to be a fairly fast reaction and slightly exothermic. Which of the following potential energy surfaces fit this description? 2. A+B General Chemistry: Chapter 14 A+B X+Y reaction coordinate Prentice-Hall © 2007

1. Energy A+B reaction coordinate 4. Energy 3. A+B X+Y reaction coordinate 24 X+Y reaction coordinate Energy The reaction between A and B is determined to be a fairly fast reaction and only slightly exothermic. Which of the following potential energy surfaces fit this description? 2. A+B General Chemistry: Chapter 14 A+B X+Y reaction coordinate Prentice-Hall © 2007

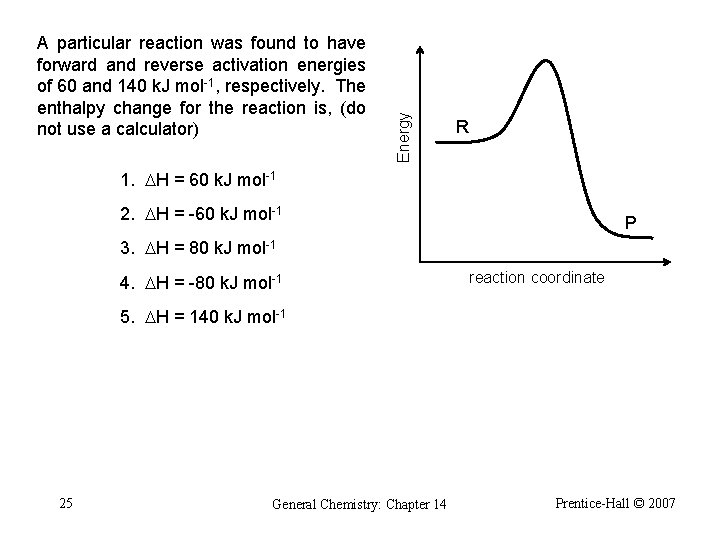
Energy A particular reaction was found to have forward and reverse activation energies of 60 and 140 k. J mol-1, respectively. The enthalpy change for the reaction is, (do not use a calculator) R 1. DH = 60 k. J mol-1 2. DH = -60 k. J mol-1 P 3. DH = 80 k. J mol-1 4. DH = -80 k. J mol-1 reaction coordinate 5. DH = 140 k. J mol-1 25 General Chemistry: Chapter 14 Prentice-Hall © 2007

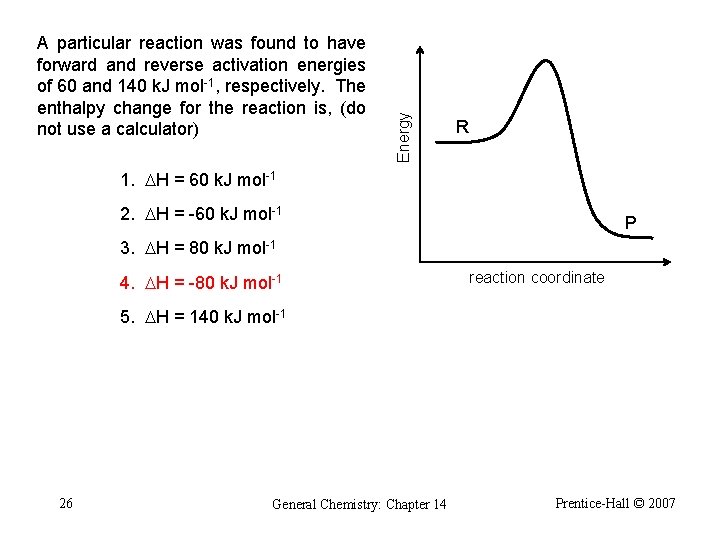
Energy A particular reaction was found to have forward and reverse activation energies of 60 and 140 k. J mol-1, respectively. The enthalpy change for the reaction is, (do not use a calculator) R 1. DH = 60 k. J mol-1 2. DH = -60 k. J mol-1 P 3. DH = 80 k. J mol-1 4. DH = -80 k. J mol-1 reaction coordinate 5. DH = 140 k. J mol-1 26 General Chemistry: Chapter 14 Prentice-Hall © 2007

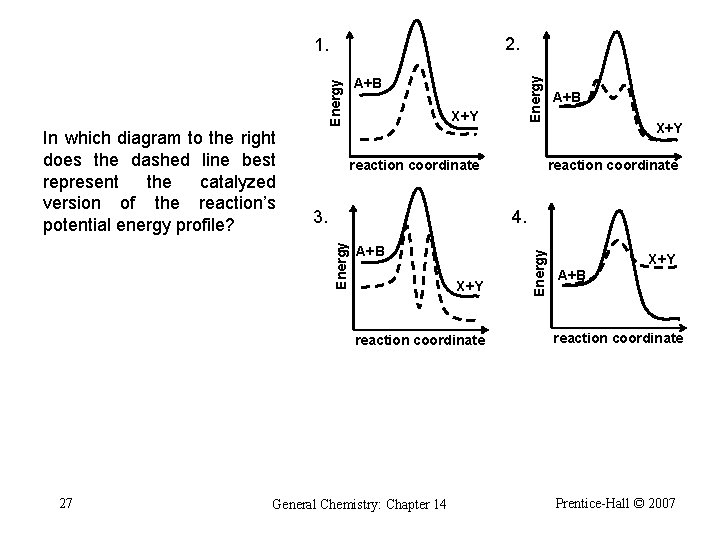
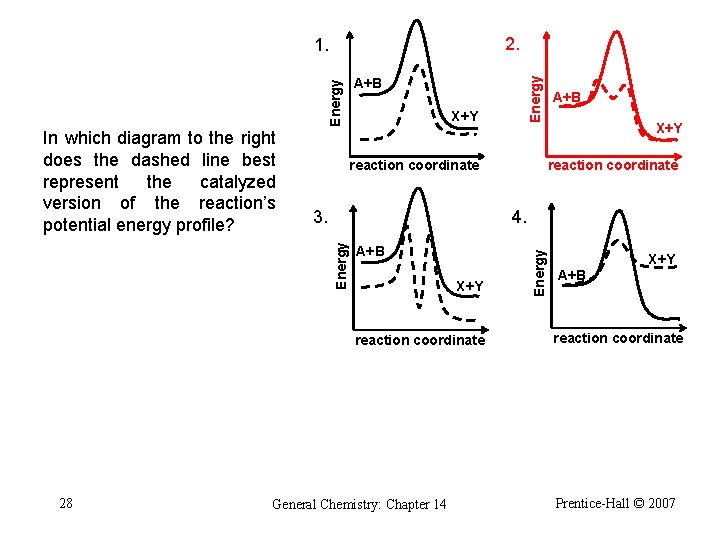
2. 1. X+Y reaction coordinate X+Y 4. A+B X+Y reaction coordinate 27 A+B reaction coordinate General Chemistry: Chapter 14 Energy 3. Energy In which diagram to the right does the dashed line best represent the catalyzed version of the reaction’s potential energy profile? Energy A+B X+Y A+B reaction coordinate Prentice-Hall © 2007

2. 1. X+Y reaction coordinate X+Y 4. A+B X+Y reaction coordinate 28 A+B reaction coordinate General Chemistry: Chapter 14 Energy 3. Energy In which diagram to the right does the dashed line best represent the catalyzed version of the reaction’s potential energy profile? Energy A+B X+Y A+B reaction coordinate Prentice-Hall © 2007

¨ Textbook End of Chapter ? ’s: ¨ P. 611 - #1, 3, 11, 13, 17, 19, ¨ 21, 33, 47, 51, 55, 100, 101, ¨ 102, 103, 104, 105 29 General Chemistry: Chapter 14 Prentice-Hall © 2007