14 4 Reaction Mechanism Steps of a Reaction

![Modification of Rate Law RATE = k [int] Written in terms of reactants- The Modification of Rate Law RATE = k [int] Written in terms of reactants- The](https://slidetodoc.com/presentation_image_h2/5f9468f412f05d61955f88db072ece13/image-15.jpg)
- Slides: 22

14. 4 Reaction Mechanism Steps of a Reaction Fred Omega Garces Chemistry 201 Miramar College 1 Reaction Mechanism June 21

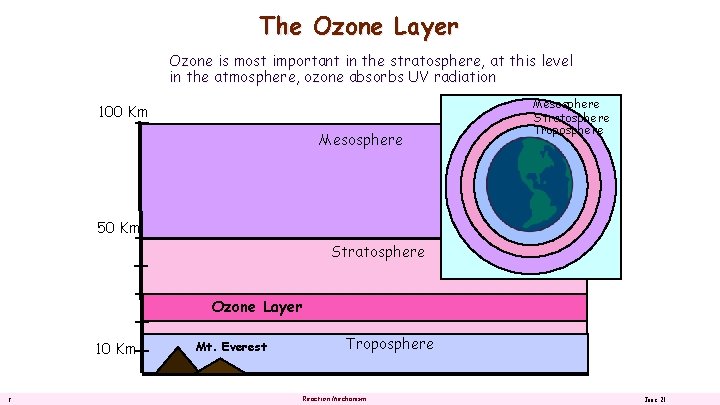
The Ozone Layer Ozone is most important in the stratosphere, at this level in the atmosphere, ozone absorbs UV radiation 100 Km Mesosphere Stratosphere Troposphere 50 Km Stratosphere Ozone Layer 10 Km 2 Mt. Everest Troposphere Reaction Mechanism June 21

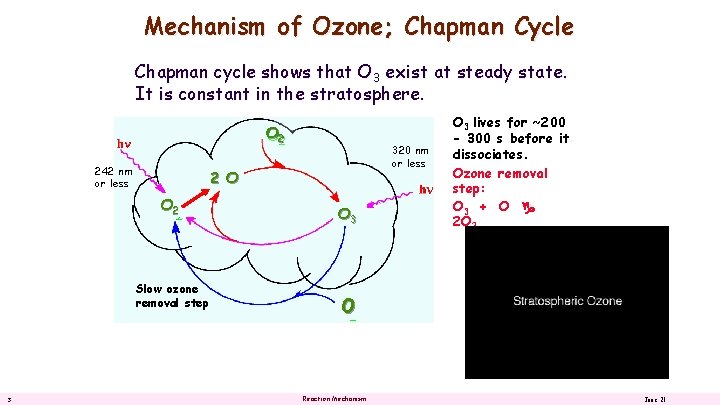
Mechanism of Ozone; Chapman Cycle Chapman cycle shows that O 3 exist at steady state. It is constant in the stratosphere. O 2 242 nm or less 2 O O 2 Slow ozone removal step 3 320 nm or less O 3 lives for ~200 - 300 s before it dissociates. Ozone removal step: O 3 + O 2 O 2 O Reaction Mechanism June 21

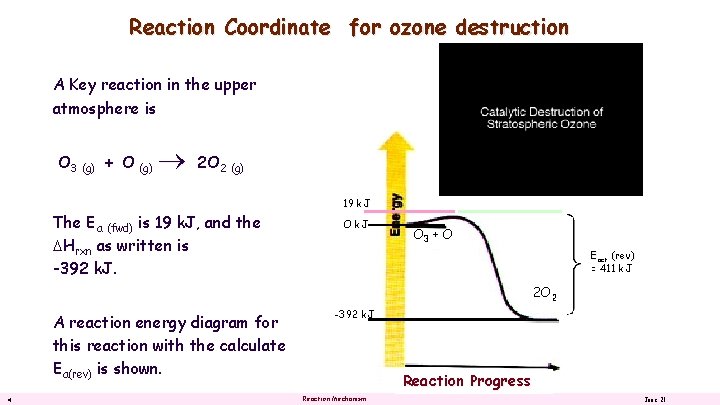
Reaction Coordinate for ozone destruction A Key reaction in the upper atmosphere is O 3 (g) + O (g) 2 O 2 (g) The Ea (fwd) is 19 k. J, and the DHrxn as written is -392 k. J. 19 k. J O 3 + O Eact (rev) = 411 k. J 2 O 2 A reaction energy diagram for this reaction with the calculate Ea(rev) is shown. 4 -392 k. J Reaction Progress Reaction Mechanism June 21

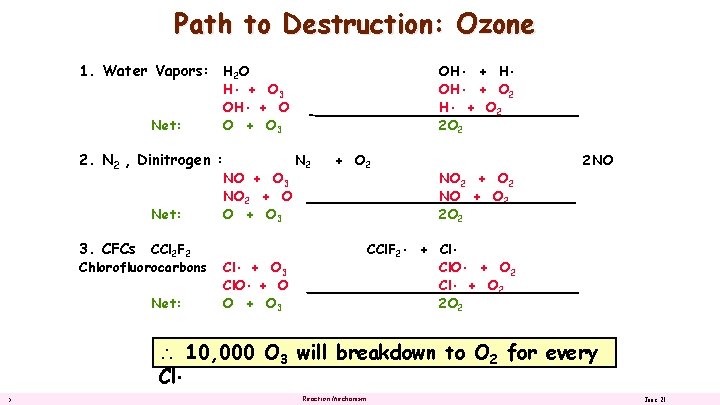
Path to Destruction: Ozone 1. Water Vapors: H 2 O Net: 2. N 2 , Dinitrogen : Net: 3. CFCs CCl 2 F 2 Chlorofluorocarbons Net: OH • + H • OH • + O 2 2 O 2 H • + O 3 OH • + O O + O 3 NO 2 + O O + O 3 N 2 + O 2 NO + O 2 2 NO CCl. F 2 • + Cl • Cl. O • + O 2 Cl • + O 2 2 O 2 Cl • + O 3 Cl. O • + O O + O 3 10, 000 O 3 will breakdown to O 2 for every Cl • 5 Reaction Mechanism June 21

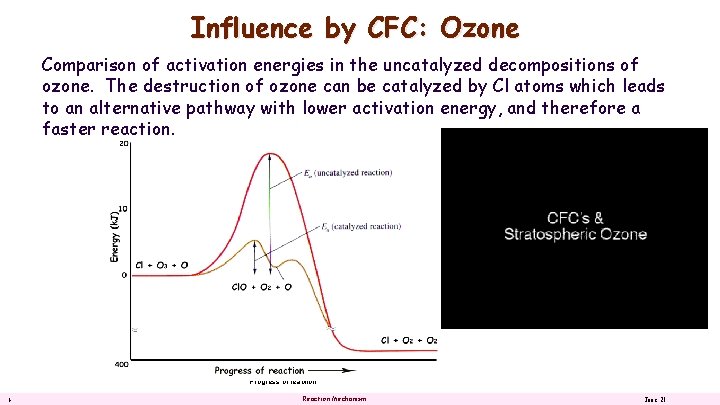
Influence by CFC: Ozone Energy (k. J) Comparison of activation energies in the uncatalyzed decompositions of ozone. The destruction of ozone can be catalyzed by Cl atoms which leads to an alternative pathway with lower activation energy, and therefore a faster reaction. Progress of reaction 6 Reaction Mechanism June 21

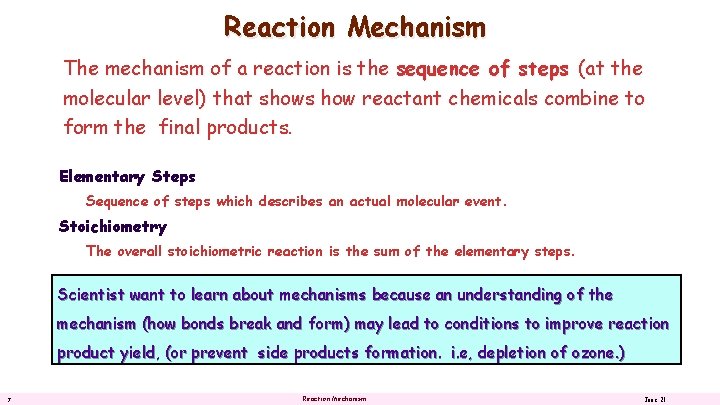
Reaction Mechanism The mechanism of a reaction is the sequence of steps (at the molecular level) that shows how reactant chemicals combine to form the final products. Elementary Steps Sequence of steps which describes an actual molecular event. Stoichiometry The overall stoichiometric reaction is the sum of the elementary steps. Scientist want to learn about mechanisms because an understanding of the mechanism (how bonds break and form) may lead to conditions to improve reaction product yield, (or prevent side products formation. i. e, depletion of ozone. ) 7 Reaction Mechanism June 21

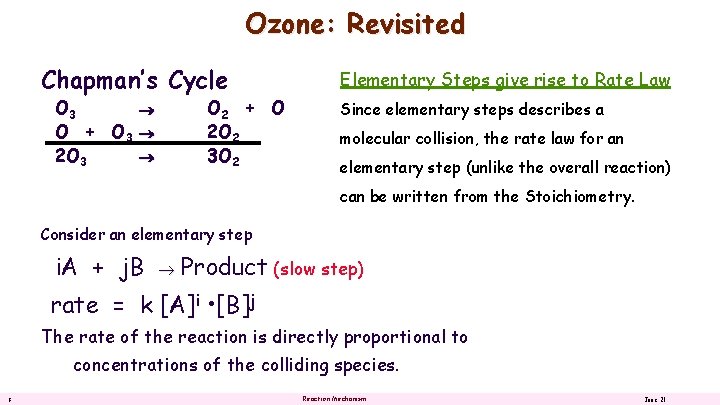
Ozone: Revisited Chapman’s Cycle O 3 O + O 3 2 O 3 O 2 + O 2 O 2 3 O 2 Elementary Steps give rise to Rate Law Since elementary steps describes a molecular collision, the rate law for an elementary step (unlike the overall reaction) can be written from the Stoichiometry. Consider an elementary step i. A + j. B Product (slow step) rate = k [A]i • [B]j The rate of the reaction is directly proportional to concentrations of the colliding species. 8 Reaction Mechanism June 21

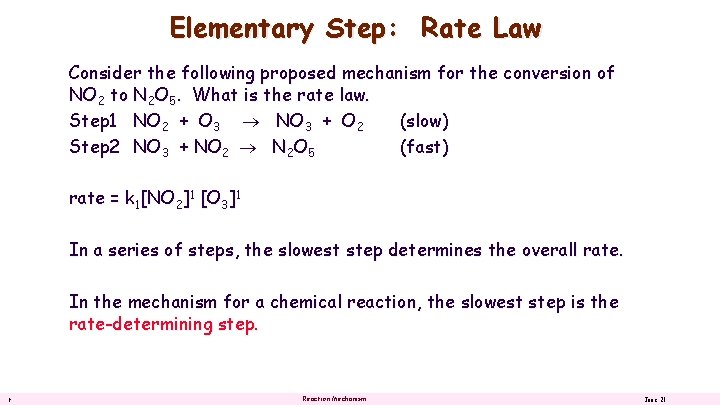
Elementary Step: Rate Law Consider the following proposed mechanism for the conversion of NO 2 to N 2 O 5. What is the rate law. Step 1 NO 2 + O 3 NO 3 + O 2 (slow) Step 2 NO 3 + NO 2 N 2 O 5 (fast) rate = k 1[NO 2]1 [O 3]1 In a series of steps, the slowest step determines the overall rate. In the mechanism for a chemical reaction, the slowest step is the rate-determining step. 9 Reaction Mechanism June 21

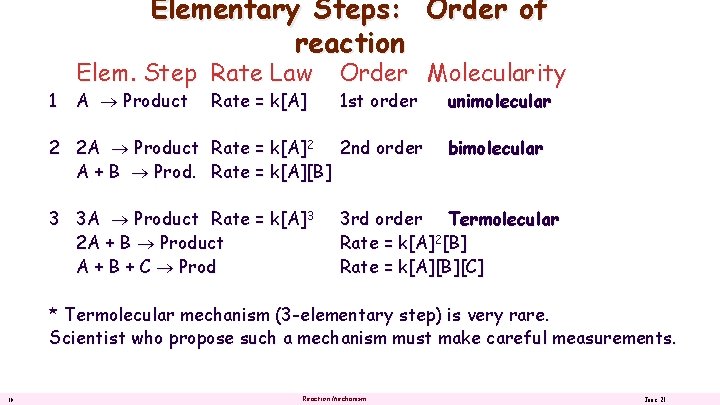
Elementary Steps: Order of reaction 1 Elem. Step Rate Law A Product Rate = k[A] Order Molecularity 1 st order 2 2 A Product Rate = k[A]2 2 nd order A + B Prod. Rate = k[A][B] 3 3 A Product Rate = k[A]3 2 A + B Product A + B + C Prod unimolecular bimolecular 3 rd order Termolecular Rate = k[A]2[B] Rate = k[A][B][C] * Termolecular mechanism (3 -elementary step) is very rare. Scientist who propose such a mechanism must make careful measurements. 10 Reaction Mechanism June 21

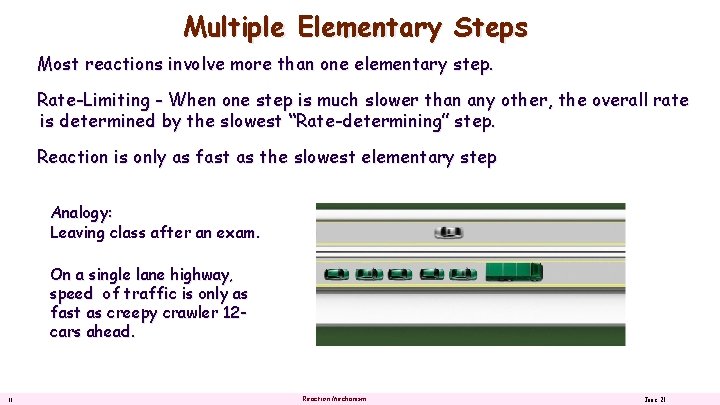
Multiple Elementary Steps Most reactions involve more than one elementary step. Rate-Limiting - When one step is much slower than any other, the overall rate is determined by the slowest “Rate-determining” step. Reaction is only as fast as the slowest elementary step Analogy: Leaving class after an exam. On a single lane highway, speed of traffic is only as fast as creepy crawler 12 cars ahead. 11 Reaction Mechanism June 21

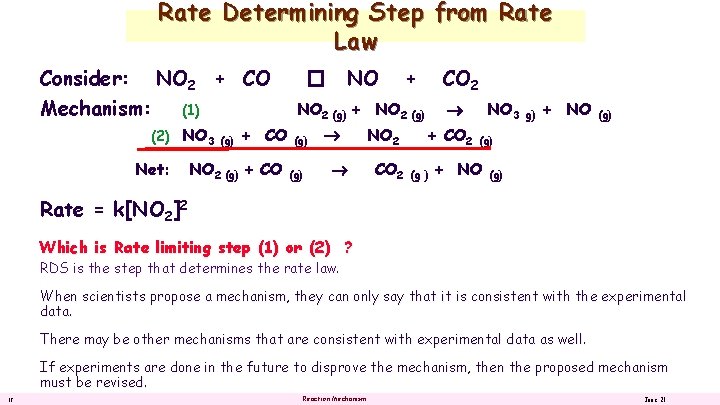
Rate Determining Step from Rate Law Consider: NO 2 + CO Mechanism: (1) (2) NO 3 Net: (g) + CO NO 2 (g) + CO NO � + CO 2 NO 2 (g) + NO 2 (g) (g) NO 2 CO 2 NO 3 + CO 2 (g ) g) + NO (g) Rate = k[NO 2]2 Which is Rate limiting step (1) or (2) ? RDS is the step that determines the rate law. When scientists propose a mechanism, they can only say that it is consistent with the experimental data. There may be other mechanisms that are consistent with experimental data as well. If experiments are done in the future to disprove the mechanism, then the proposed mechanism must be revised. 12 Reaction Mechanism June 21

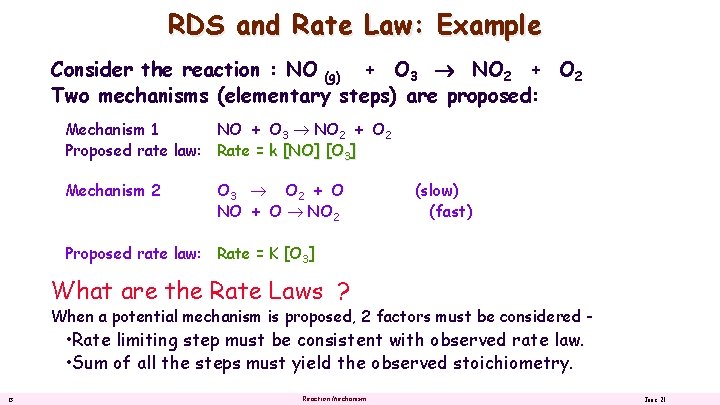
RDS and Rate Law: Example Consider the reaction : NO (g) + O 3 NO 2 + O 2 Two mechanisms (elementary steps) are proposed: Mechanism 1 NO + O 3 NO 2 + O 2 Proposed rate law: Rate = k [NO] [O 3] Mechanism 2 O 3 O 2 + O NO + O NO 2 (slow) (fast) Proposed rate law: Rate = K [O 3] What are the Rate Laws ? When a potential mechanism is proposed, 2 factors must be considered - • Rate limiting step must be consistent with observed rate law. • Sum of all the steps must yield the observed stoichiometry. 13 Reaction Mechanism June 21

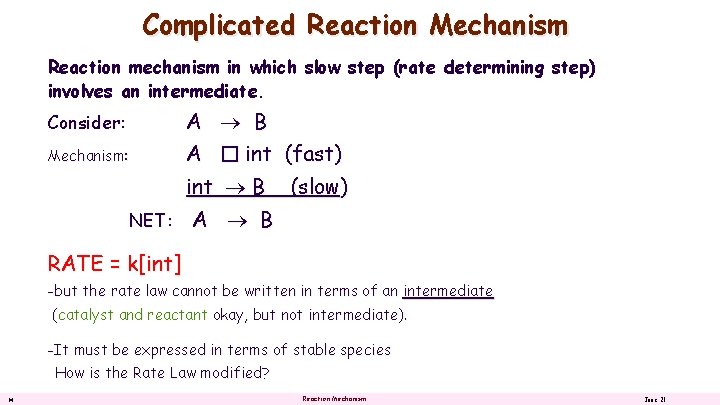
Complicated Reaction Mechanism Reaction mechanism in which slow step (rate determining step) involves an intermediate. Consider: A B Mechanism: A � int (fast) int B NET: (slow) A B RATE = k[int] -but the rate law cannot be written in terms of an intermediate (catalyst and reactant okay, but not intermediate). -It must be expressed in terms of stable species How is the Rate Law modified? 14 Reaction Mechanism June 21
![Modification of Rate Law RATE k int Written in terms of reactants The Modification of Rate Law RATE = k [int] Written in terms of reactants- The](https://slidetodoc.com/presentation_image_h2/5f9468f412f05d61955f88db072ece13/image-15.jpg)
Modification of Rate Law RATE = k [int] Written in terms of reactants- The rate law is now expressed in terms of the reactant. 15 Reaction Mechanism June 21

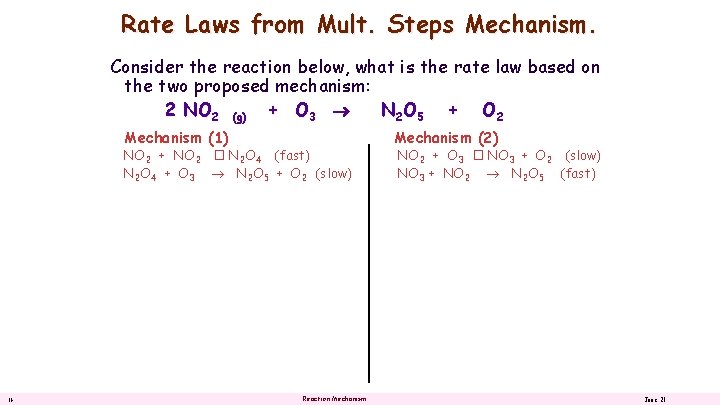
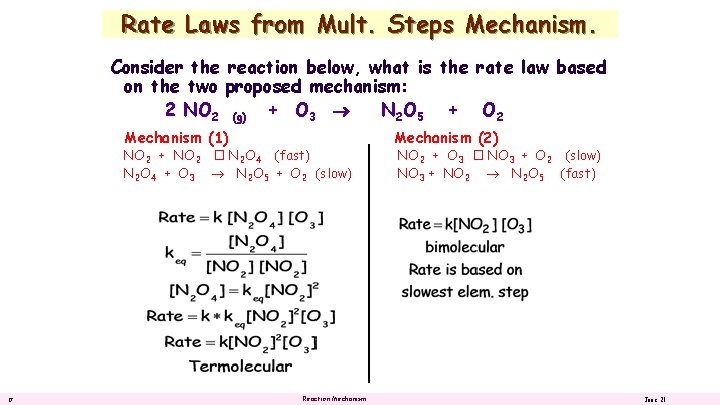
Rate Laws from Mult. Steps Mechanism. Consider the reaction below, what is the rate law based on the two proposed mechanism: 2 NO 2 (g) + O 3 N 2 O 5 + O 2 Mechanism (1) NO 2 + NO 2 � N 2 O 4 (fast) N 2 O 4 + O 3 N 2 O 5 + O 2 (slow) 16 Reaction Mechanism (2) NO 2 + O 3 � NO 3 + O 2 (slow) NO 3 + NO 2 N 2 O 5 (fast) June 21

Rate Laws from Mult. Steps Mechanism. Consider the reaction below, what is the rate law based on the two proposed mechanism: 2 NO 2 (g) + O 3 N 2 O 5 + O 2 Mechanism (1) NO 2 + NO 2 � N 2 O 4 (fast) N 2 O 4 + O 3 N 2 O 5 + O 2 (slow) 17 Reaction Mechanism (2) NO 2 + O 3 � NO 3 + O 2 (slow) NO 3 + NO 2 N 2 O 5 (fast) June 21

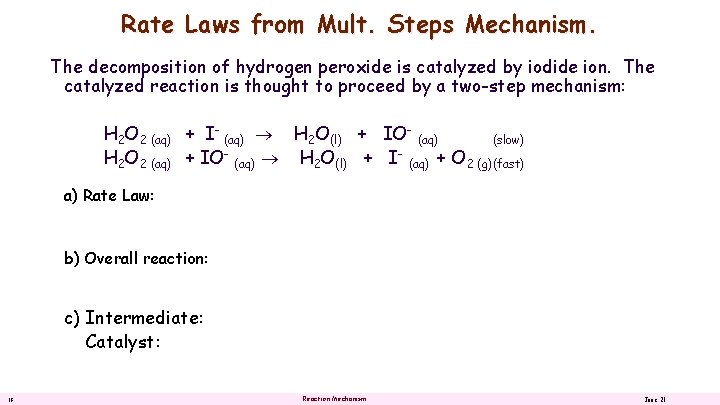
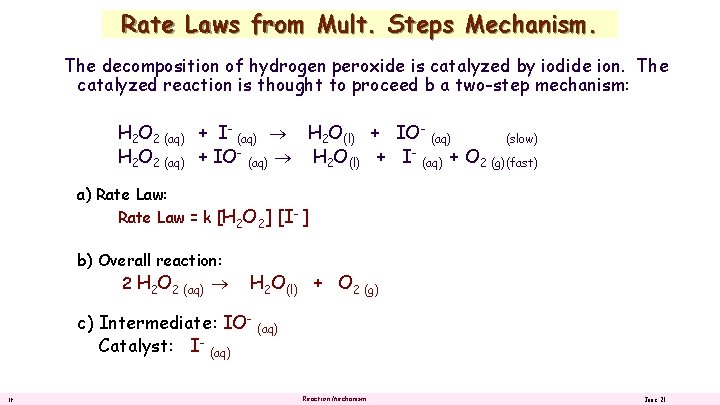
Rate Laws from Mult. Steps Mechanism. The decomposition of hydrogen peroxide is catalyzed by iodide ion. The catalyzed reaction is thought to proceed by a two-step mechanism: H 2 O 2 (aq) + I- (aq) H 2 O(l) + IO- (aq) (slow) H 2 O 2 (aq) + IO (aq) H 2 O(l) + I (aq) + O 2 (g)(fast) a) Rate Law: b) Overall reaction: c) Intermediate: Catalyst: 18 Reaction Mechanism June 21

Rate Laws from Mult. Steps Mechanism. The decomposition of hydrogen peroxide is catalyzed by iodide ion. The catalyzed reaction is thought to proceed b a two-step mechanism: H 2 O 2 (aq) + I- (aq) H 2 O(l) + IO- (aq) (slow) H 2 O 2 (aq) + IO- (aq) H 2 O(l) + I- (aq) + O 2 (g)(fast) a) Rate Law: Rate Law = k [H 2 O 2] [I- ] b) Overall reaction: 2 H 2 O 2 (aq) H 2 O(l) + O 2 (g) c) Intermediate: IOCatalyst: I- (aq) 19 (aq) Reaction Mechanism June 21

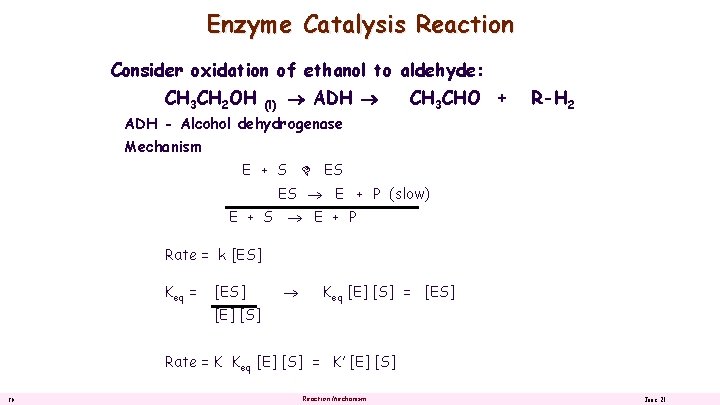
Enzyme Catalysis Reaction Consider oxidation of ethanol to aldehyde: CH 3 CH 2 OH (l) ADH - Alcohol dehydrogenase CH 3 CHO + R-H 2 Mechanism E + S ES ES E + P (slow) E + S E + P Rate = k [ES] Keq = [ES] Keq [E] [S] = [ES] [E] [S] Rate = K Keq [E] [S] = K’ [E] [S] 20 Reaction Mechanism June 21

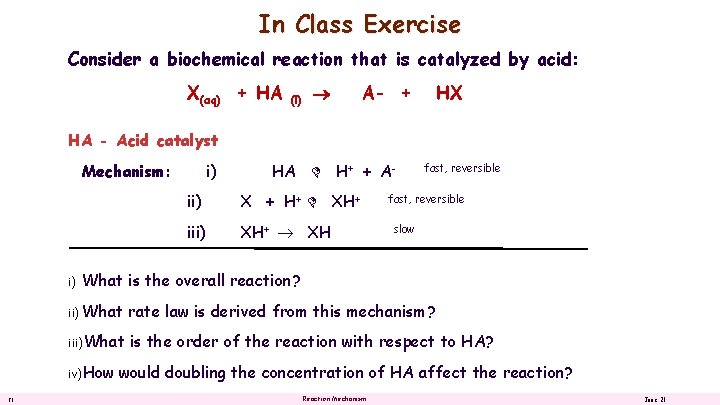
In Class Exercise Consider a biochemical reaction that is catalyzed by acid: X(aq) + HA (l) A- + HX HA - Acid catalyst Mechanism: i) HA H+ + A- ii) X + H+ XH+ iii) XH+ XH fast, reversible slow What is the overall reaction? ii) What rate law is derived from this mechanism? iii)What is the order of the reaction with respect to HA? iv)How 21 i) would doubling the concentration of HA affect the reaction? Reaction Mechanism June 21

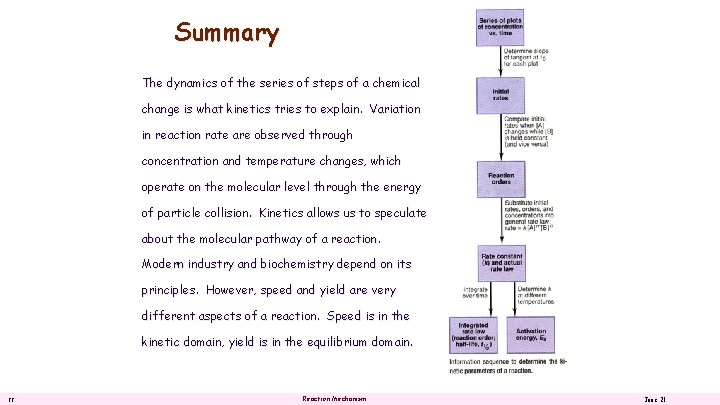
Summary The dynamics of the series of steps of a chemical change is what kinetics tries to explain. Variation in reaction rate are observed through concentration and temperature changes, which operate on the molecular level through the energy of particle collision. Kinetics allows us to speculate about the molecular pathway of a reaction. Modern industry and biochemistry depend on its principles. However, speed and yield are very different aspects of a reaction. Speed is in the kinetic domain, yield is in the equilibrium domain. 22 Reaction Mechanism June 21