13 8 Preparation of Phenols 2 million tons



- Slides: 29

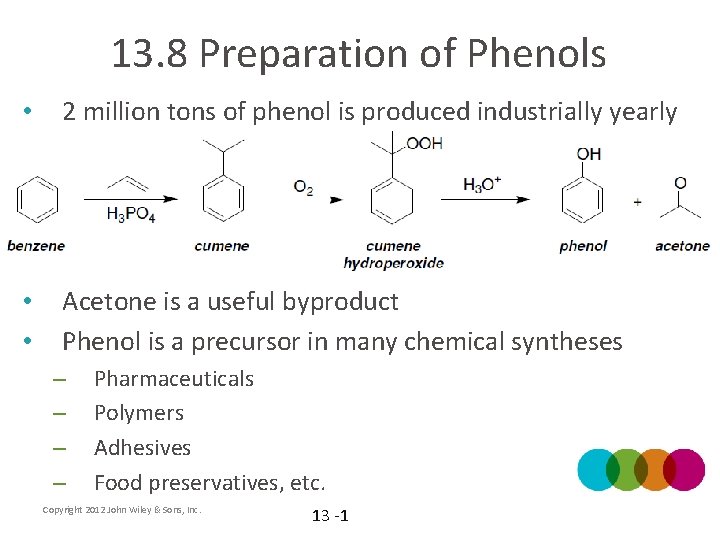
13. 8 Preparation of Phenols • 2 million tons of phenol is produced industrially yearly • • Acetone is a useful byproduct Phenol is a precursor in many chemical syntheses – – Pharmaceuticals Polymers Adhesives Food preservatives, etc. Copyright 2012 John Wiley & Sons, Inc. 13 -1

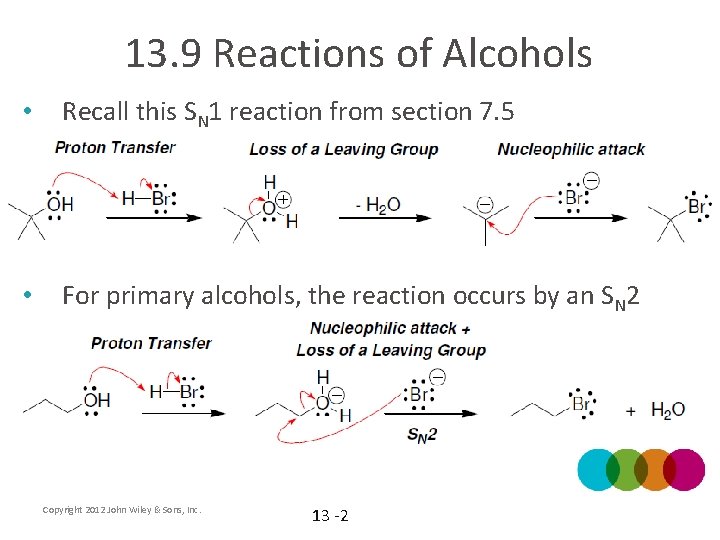
13. 9 Reactions of Alcohols • Recall this SN 1 reaction from section 7. 5 • For primary alcohols, the reaction occurs by an SN 2 Copyright 2012 John Wiley & Sons, Inc. 13 -2

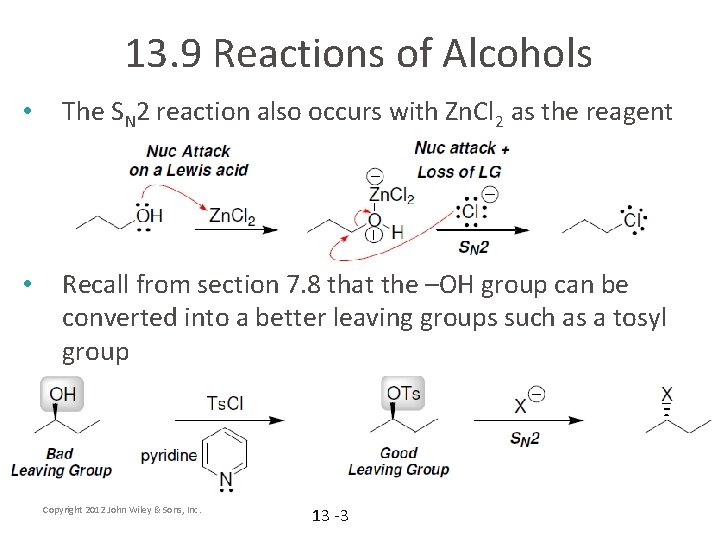
13. 9 Reactions of Alcohols • The SN 2 reaction also occurs with Zn. Cl 2 as the reagent • Recall from section 7. 8 that the –OH group can be converted into a better leaving groups such as a tosyl group Copyright 2012 John Wiley & Sons, Inc. 13 -3

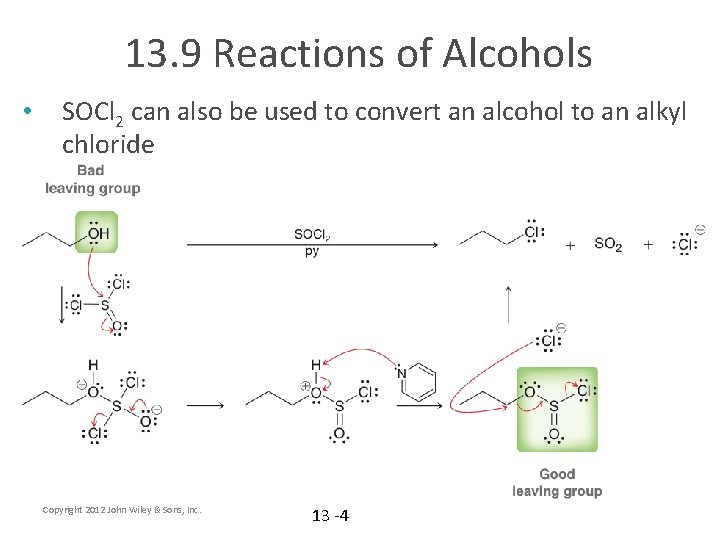
13. 9 Reactions of Alcohols • SOCl 2 can also be used to convert an alcohol to an alkyl chloride Copyright 2012 John Wiley & Sons, Inc. 13 -4

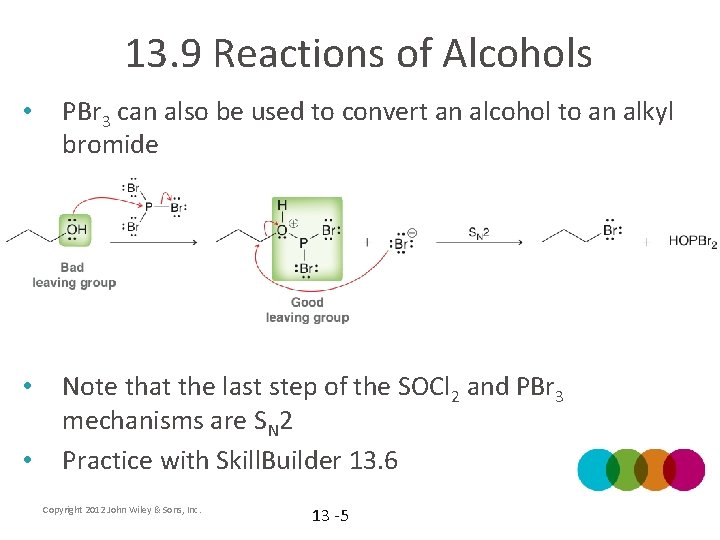
13. 9 Reactions of Alcohols • PBr 3 can also be used to convert an alcohol to an alkyl bromide • Note that the last step of the SOCl 2 and PBr 3 mechanisms are SN 2 Practice with Skill. Builder 13. 6 • Copyright 2012 John Wiley & Sons, Inc. 13 -5

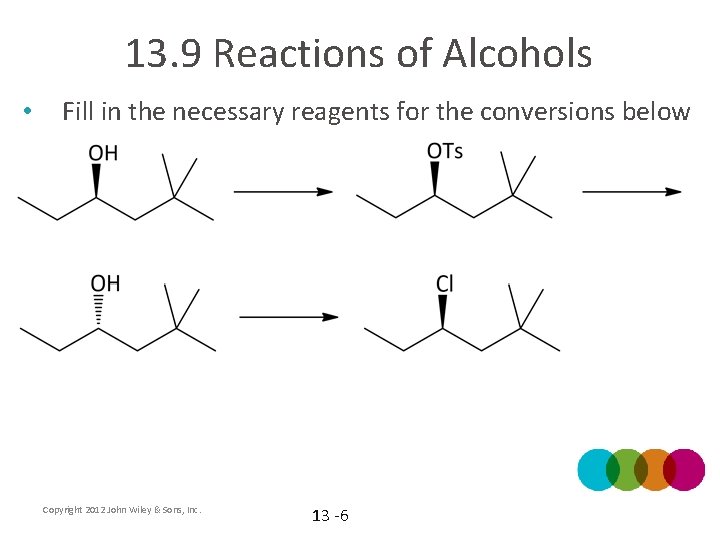
13. 9 Reactions of Alcohols • Fill in the necessary reagents for the conversions below Copyright 2012 John Wiley & Sons, Inc. 13 -6

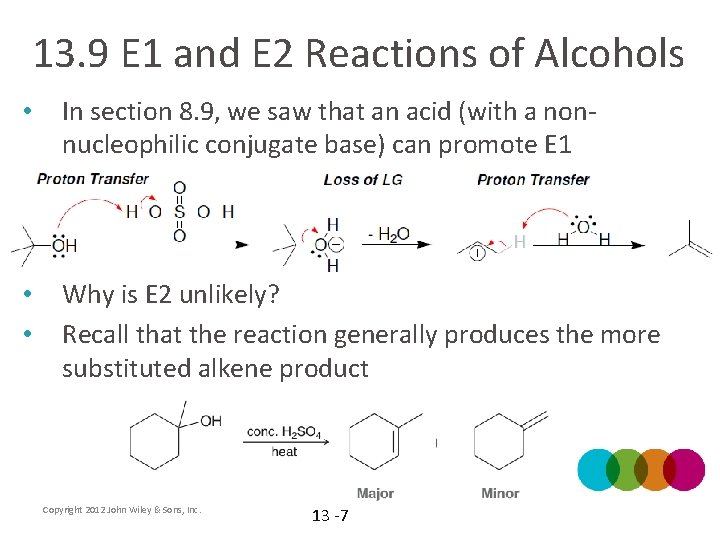
13. 9 E 1 and E 2 Reactions of Alcohols • In section 8. 9, we saw that an acid (with a nonnucleophilic conjugate base) can promote E 1 • • Why is E 2 unlikely? Recall that the reaction generally produces the more substituted alkene product Copyright 2012 John Wiley & Sons, Inc. 13 -7

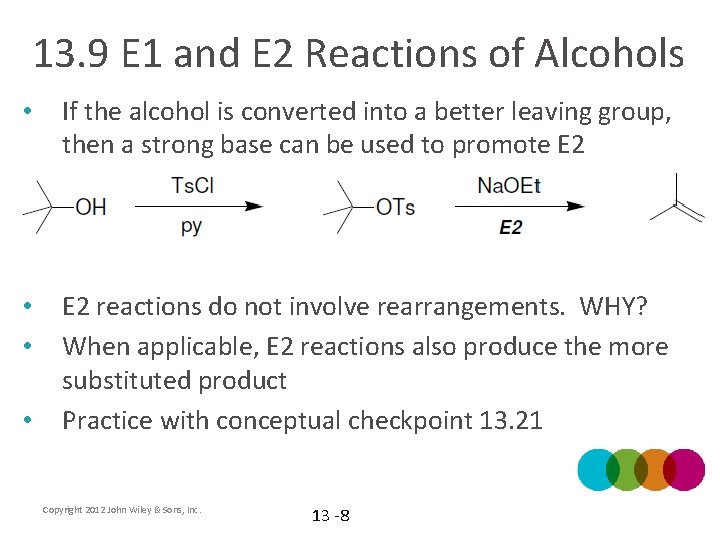
13. 9 E 1 and E 2 Reactions of Alcohols • If the alcohol is converted into a better leaving group, then a strong base can be used to promote E 2 • • E 2 reactions do not involve rearrangements. WHY? When applicable, E 2 reactions also produce the more substituted product Practice with conceptual checkpoint 13. 21 • Copyright 2012 John Wiley & Sons, Inc. 13 -8

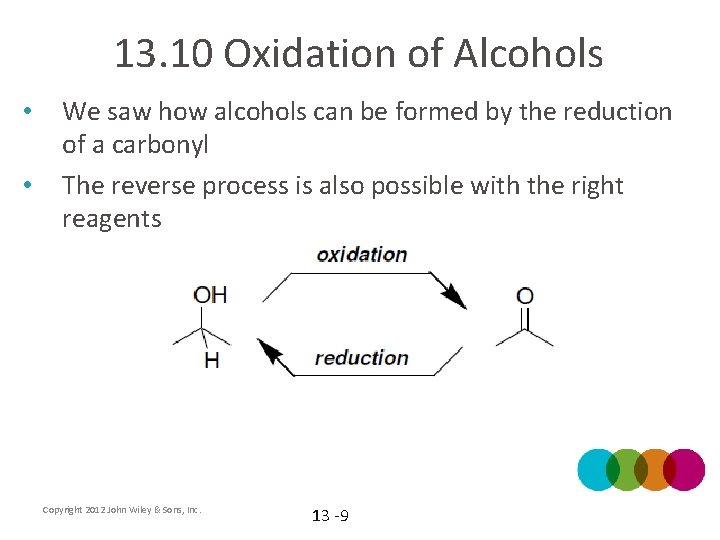
13. 10 Oxidation of Alcohols • • We saw how alcohols can be formed by the reduction of a carbonyl The reverse process is also possible with the right reagents Copyright 2012 John Wiley & Sons, Inc. 13 -9

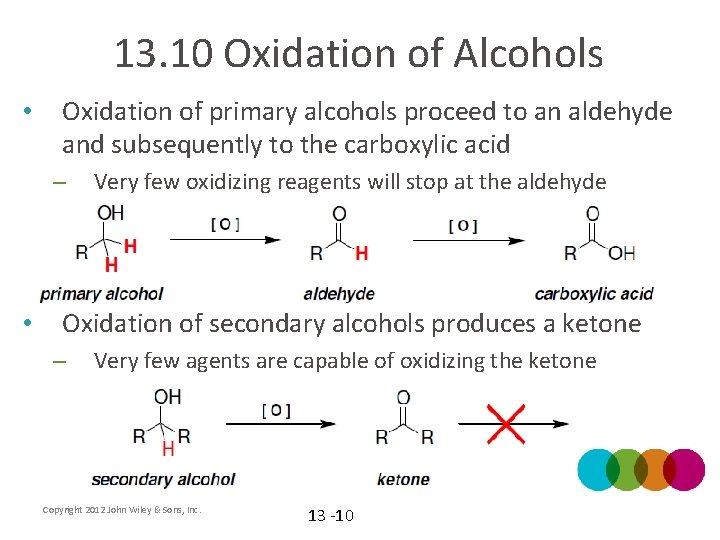
13. 10 Oxidation of Alcohols • Oxidation of primary alcohols proceed to an aldehyde and subsequently to the carboxylic acid – • Very few oxidizing reagents will stop at the aldehyde Oxidation of secondary alcohols produces a ketone – Very few agents are capable of oxidizing the ketone Copyright 2012 John Wiley & Sons, Inc. 13 -10

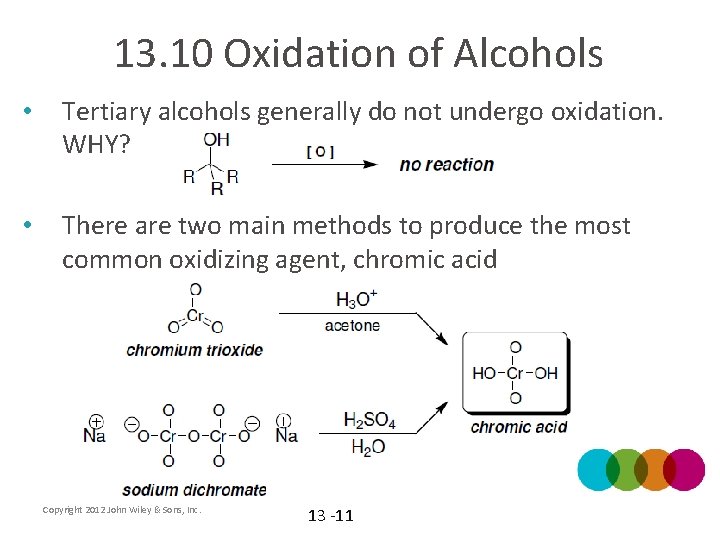
13. 10 Oxidation of Alcohols • Tertiary alcohols generally do not undergo oxidation. WHY? • There are two main methods to produce the most common oxidizing agent, chromic acid Copyright 2012 John Wiley & Sons, Inc. 13 -11

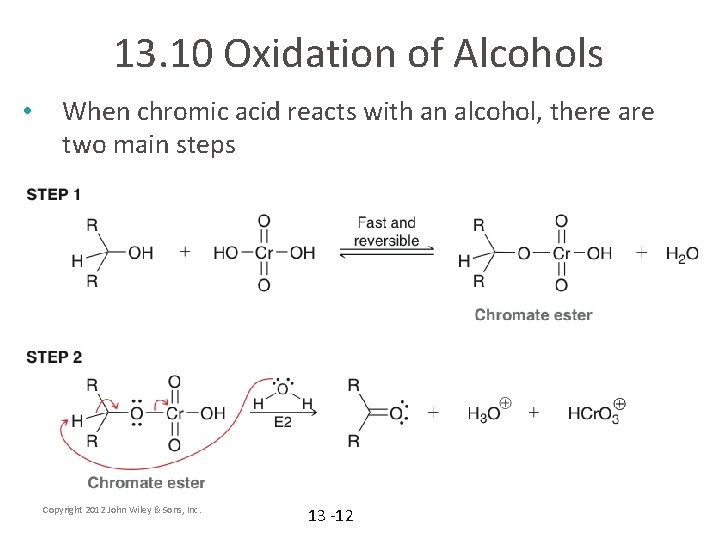
13. 10 Oxidation of Alcohols • When chromic acid reacts with an alcohol, there are two main steps Copyright 2012 John Wiley & Sons, Inc. 13 -12

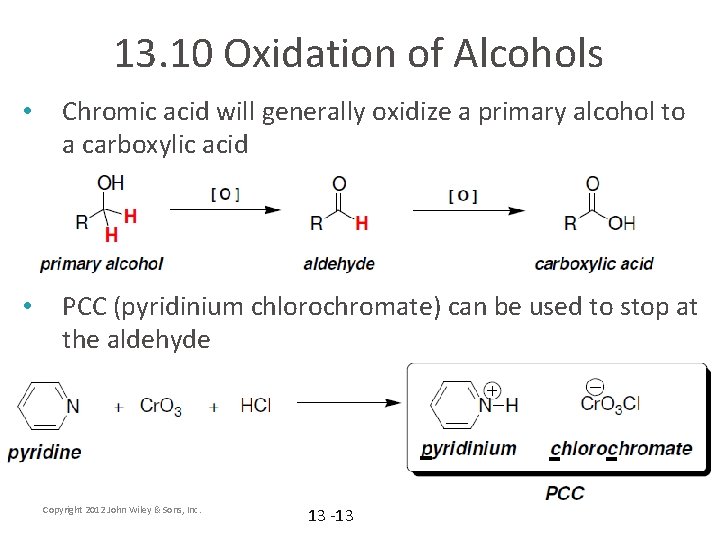
13. 10 Oxidation of Alcohols • Chromic acid will generally oxidize a primary alcohol to a carboxylic acid • PCC (pyridinium chlorochromate) can be used to stop at the aldehyde Copyright 2012 John Wiley & Sons, Inc. 13 -13

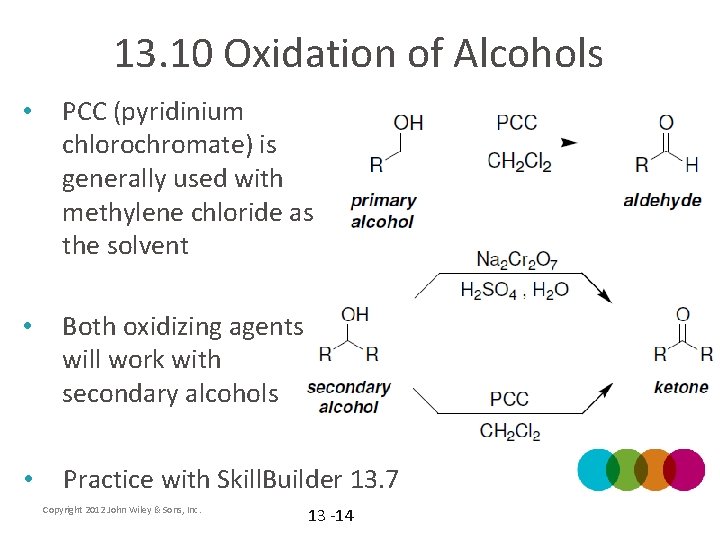
13. 10 Oxidation of Alcohols • PCC (pyridinium chlorochromate) is generally used with methylene chloride as the solvent • Both oxidizing agents will work with secondary alcohols • Practice with Skill. Builder 13. 7 Copyright 2012 John Wiley & Sons, Inc. 13 -14

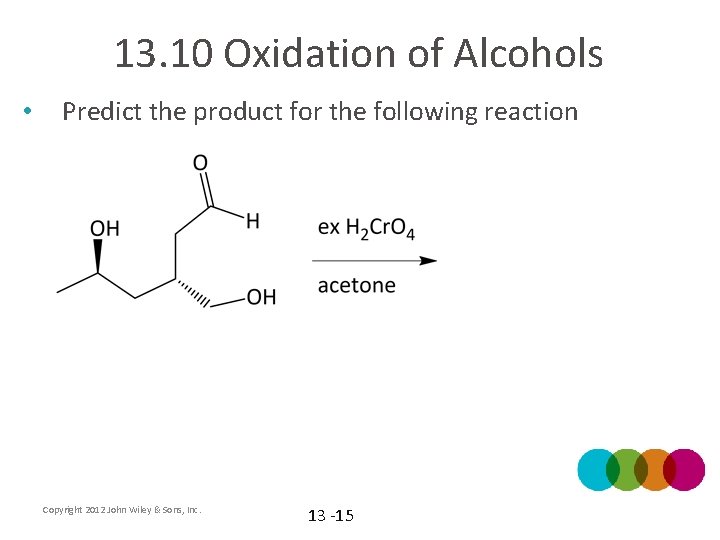
13. 10 Oxidation of Alcohols • Predict the product for the following reaction Copyright 2012 John Wiley & Sons, Inc. 13 -15

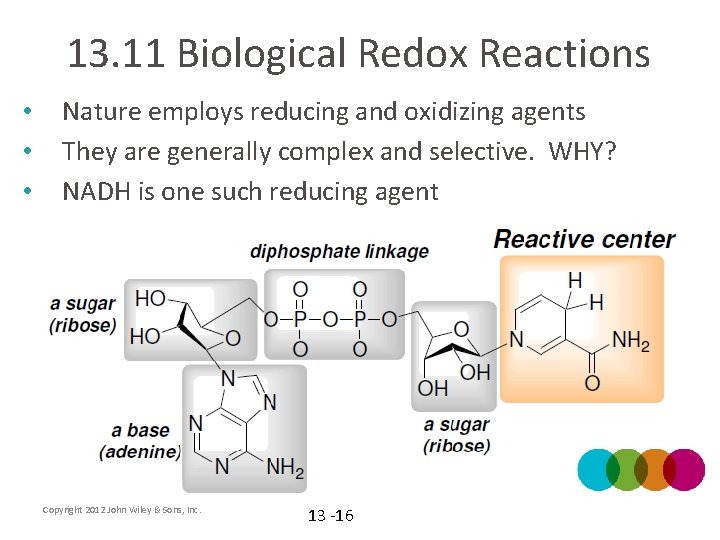
13. 11 Biological Redox Reactions • • • Nature employs reducing and oxidizing agents They are generally complex and selective. WHY? NADH is one such reducing agent Copyright 2012 John Wiley & Sons, Inc. 13 -16

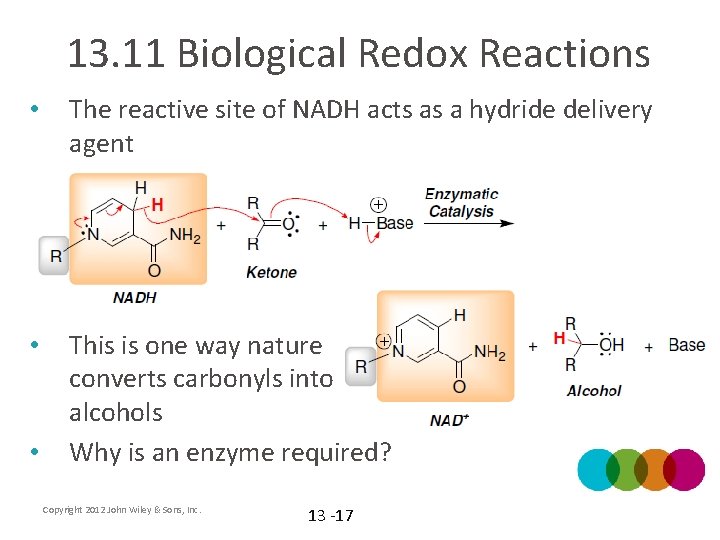
13. 11 Biological Redox Reactions • The reactive site of NADH acts as a hydride delivery agent • This is one way nature converts carbonyls into alcohols Why is an enzyme required? • Copyright 2012 John Wiley & Sons, Inc. 13 -17

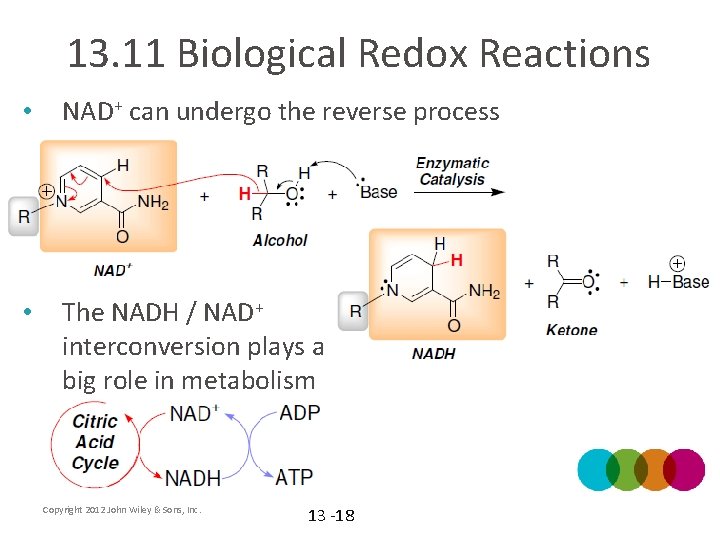
13. 11 Biological Redox Reactions • NAD+ can undergo the reverse process • The NADH / NAD+ interconversion plays a big role in metabolism Copyright 2012 John Wiley & Sons, Inc. 13 -18

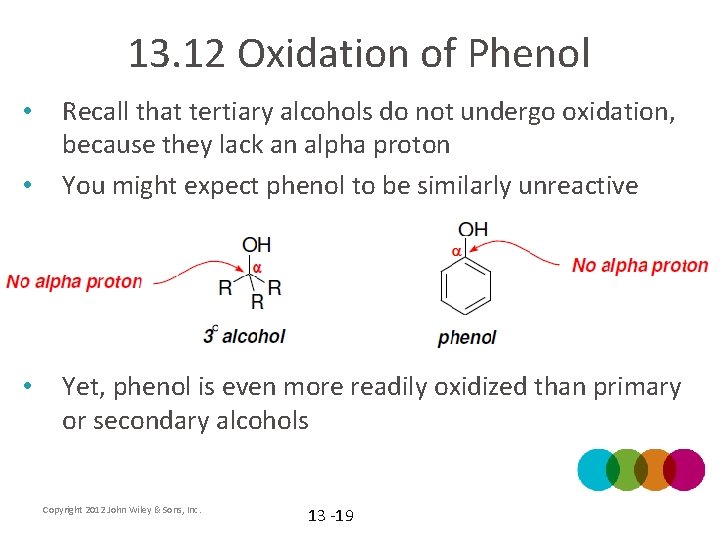
13. 12 Oxidation of Phenol • • • Recall that tertiary alcohols do not undergo oxidation, because they lack an alpha proton You might expect phenol to be similarly unreactive Yet, phenol is even more readily oxidized than primary or secondary alcohols Copyright 2012 John Wiley & Sons, Inc. 13 -19

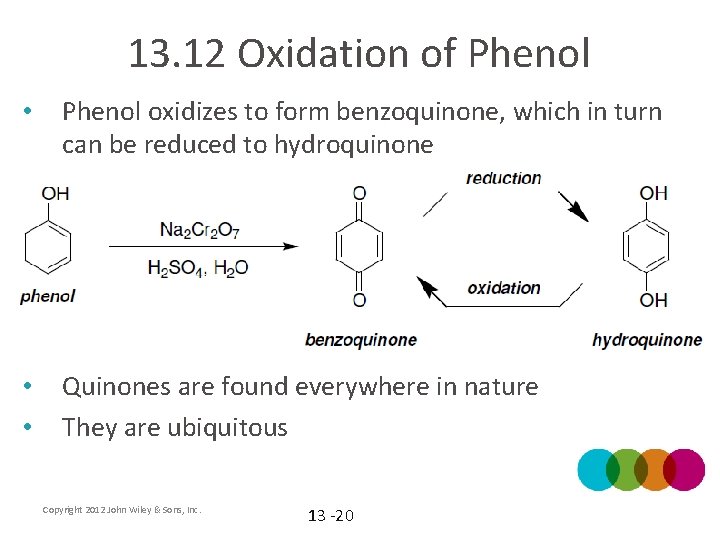
13. 12 Oxidation of Phenol • Phenol oxidizes to form benzoquinone, which in turn can be reduced to hydroquinone • • Quinones are found everywhere in nature They are ubiquitous Copyright 2012 John Wiley & Sons, Inc. 13 -20

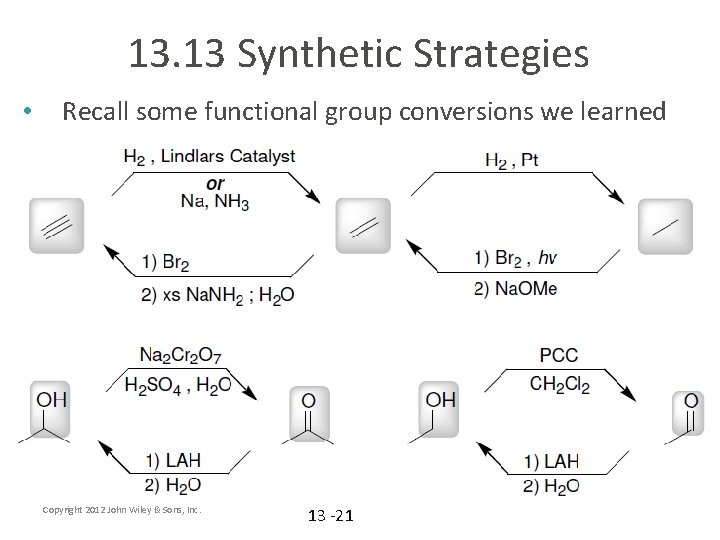
13. 13 Synthetic Strategies • Recall some functional group conversions we learned Copyright 2012 John Wiley & Sons, Inc. 13 -21

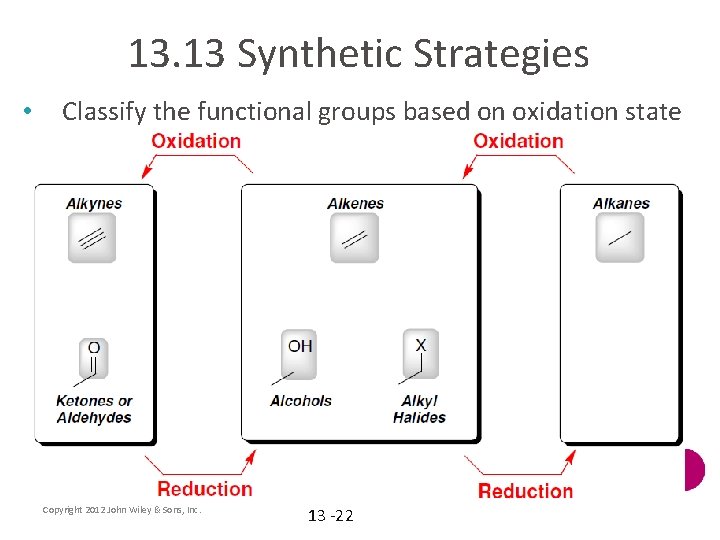
13. 13 Synthetic Strategies • Classify the functional groups based on oxidation state Copyright 2012 John Wiley & Sons, Inc. 13 -22

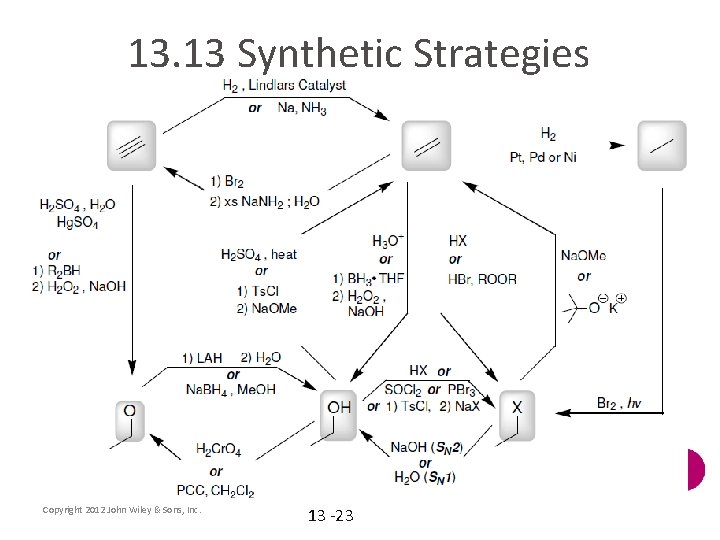
13. 13 Synthetic Strategies Copyright 2012 John Wiley & Sons, Inc. 13 -23

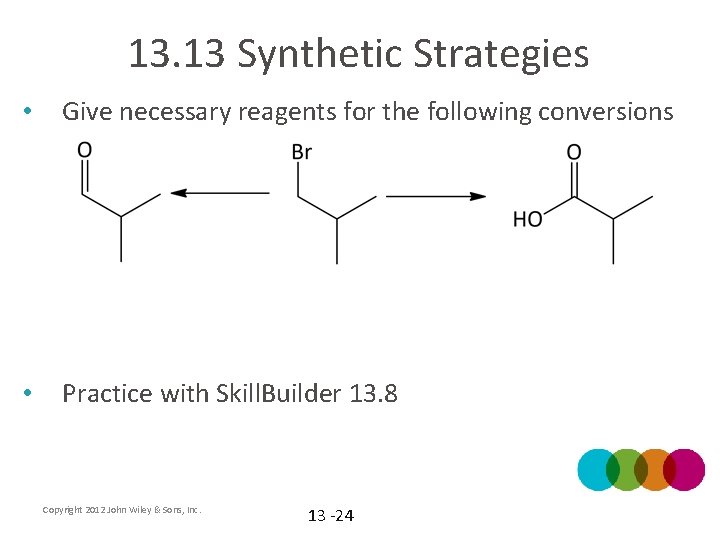
13. 13 Synthetic Strategies • Give necessary reagents for the following conversions • Practice with Skill. Builder 13. 8 Copyright 2012 John Wiley & Sons, Inc. 13 -24

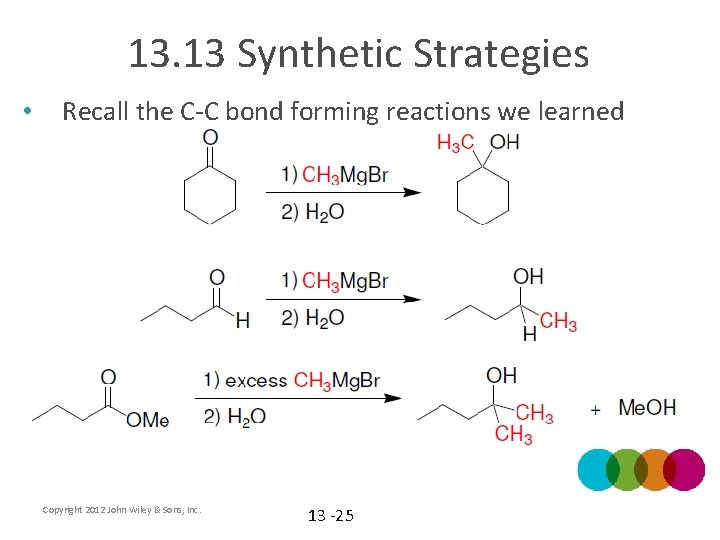
13. 13 Synthetic Strategies • Recall the C-C bond forming reactions we learned Copyright 2012 John Wiley & Sons, Inc. 13 -25

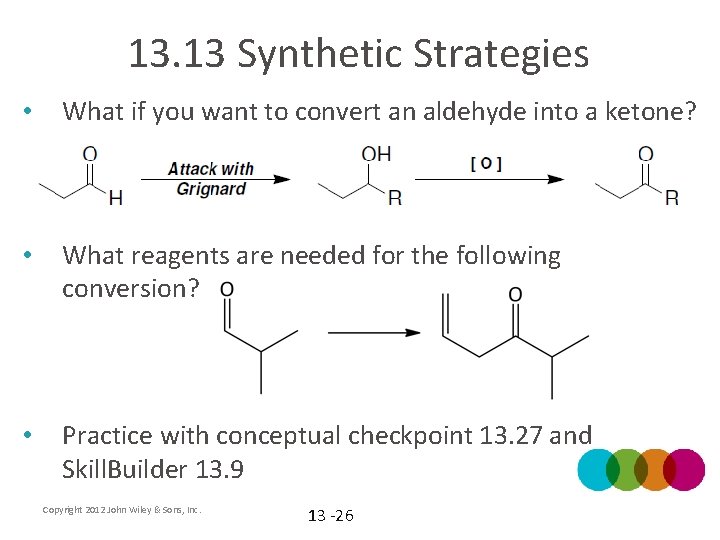
13. 13 Synthetic Strategies • What if you want to convert an aldehyde into a ketone? • What reagents are needed for the following conversion? • Practice with conceptual checkpoint 13. 27 and Skill. Builder 13. 9 Copyright 2012 John Wiley & Sons, Inc. 13 -26

Study Guide for Sections 13. 8 -13. 13 DAY 6, Terms to know: Sections 13. 8 -13. 13 PCC, benzoquinone, hydroquinone, DAY 6, Specific outcomes and skills that may be tested on exam 1: Sections 13. 8 -13. 13 • Given products and reactant(s), be able to choose appropriate reagents for any of the reactions discussed that alcohols undergo • Given a specific alcohol and specific reagents, be able to predict the products of the reaction and draw a complete mechanism for any of the reaction we discussed • Be able to solve 1, 2, or 3 step syntheses involving any of the reactions discussed up to this point • Given some reagents and/or intermediates, be able to solve syntheses longer than 3 steps • Be able to explain regio- and stereo- outcomes predicted in syntheses • Be able to perform a retrosynthetic analysis to design a synthesis for a specific product

Extra Practice Problems for Sections 13. 8 -13. 13 Complete these problems outside of class until you are confident you have learned the SKILLS in this section outlined on the study guide and we will review some of them next class period. 13. 19 13. 20 13. 21 13. 22 13. 23 13. 24 13. 26 13. 27 13. 28 13. 29 13. 35 13. 37 a, b, c, and e 13. 38 13. 39 13. 40 13. 41 13. 43 13. 44 13. 47 13. 49 b, d-f 13. 50 13. 51

Day 7: EXAM 1 Prep for Day 8 Must Watch videos: https: //www. youtube. com/watch? v=ro. UGDG 1 rh. PI (naming ethers, Khan) https: //www. youtube. com/watch? v=UGT-FATdv. JM (ethers, FLC) https: //www. youtube. com/watch? v=t. SBX 4_HCHVk (epoxides, FLC) https: //www. youtube. com/watch? v=Ul. Jm. X 9 SLGRw (properties of ethers, Khan) https: //www. youtube. com/watch? v=1 k 6 MUe. M-p. Eo (cleavage of ethers, Khan) https: //www. youtube. com/watch? v=5 d 63 YMUxc. S 4 (naming cyclic ethers and epoxides, Khan) Other helpful videos: https: //www. youtube. com/watch? v=tk. Ih. M 6 D 3 Ojs (ether synthesis, moballer 12) https: //www. youtube. com/watch? v=HWj. OHSM 0 S_I (naming ethers, Leah) http: //ocw. uci. edu/lectures/chemistry_51 b_organic_chemistry_lec_06. html (ethers, UC-Irvine) start 20 minutes in. http: //ocw. uci. edu/lectures/chem_51 b_lec_02_organic_chemistry_alcohols_ethers_and_expodites_part_1. html (alcohols, UC-Irvine) start 12 minutes in. http: //ocw. uci. edu/lectures/chem_51 b_lec_03_organic_chemistry_alcohols_ethers_and_expodites_part_2. html (alcohols, UC-Irvine) start 14 minutes in. Read Sections 14. 1 -14. 7