13 8 NOTES Naming Branching Alkanes Alkanes Revisited

13. 8 – NOTES Naming Branching Alkanes

• Alkanes Revisited • Straight-chain alkanes • Each carbon atom is linked to 1 or 2 carbons depending on location in the chain
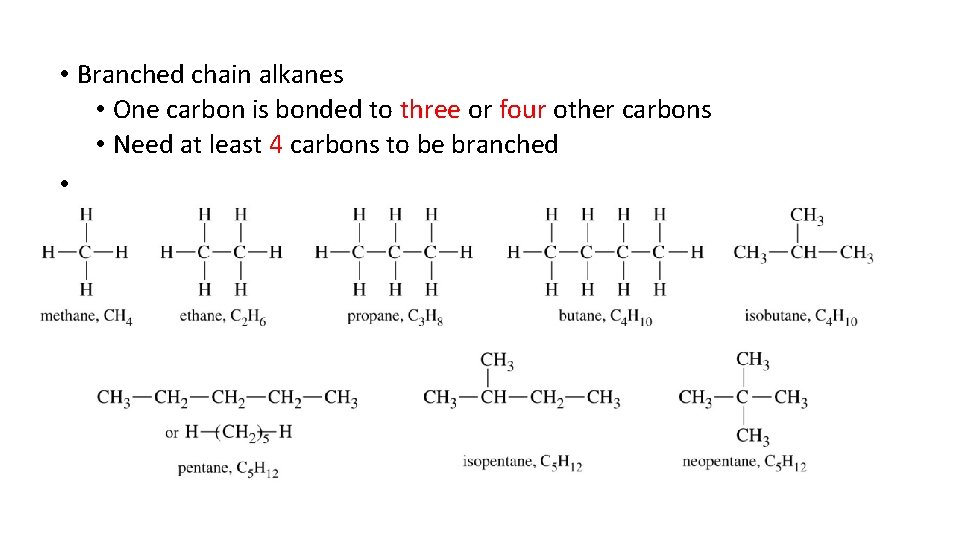
• Branched chain alkanes • One carbon is bonded to three or four other carbons • Need at least 4 carbons to be branched •
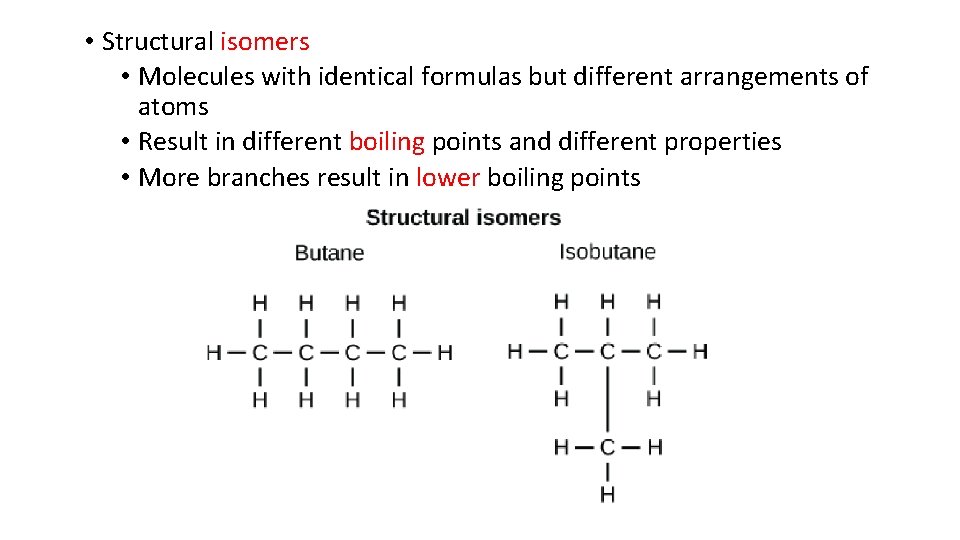
• Structural isomers • Molecules with identical formulas but different arrangements of atoms • Result in different boiling points and different properties • More branches result in lower boiling points
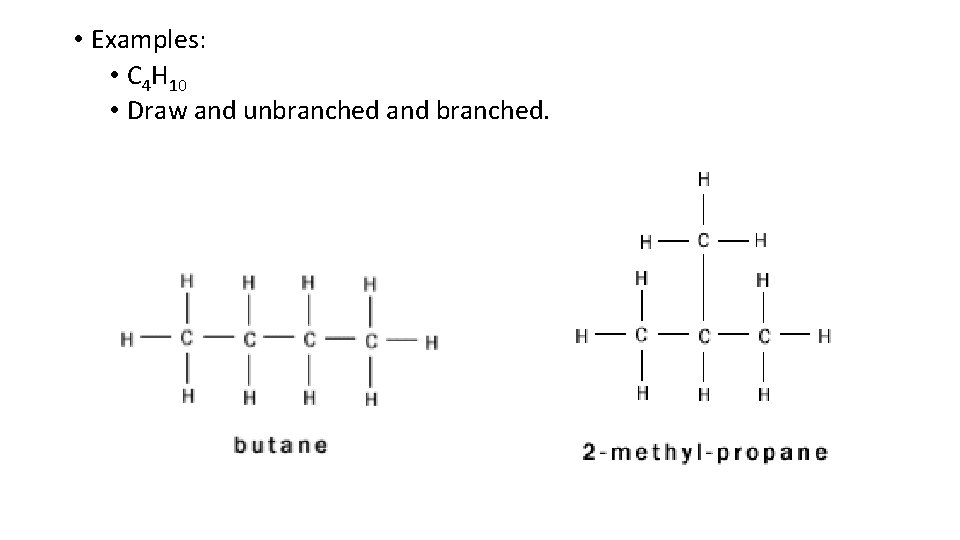
• Examples: • C 4 H 10 • Draw and unbranched and branched.
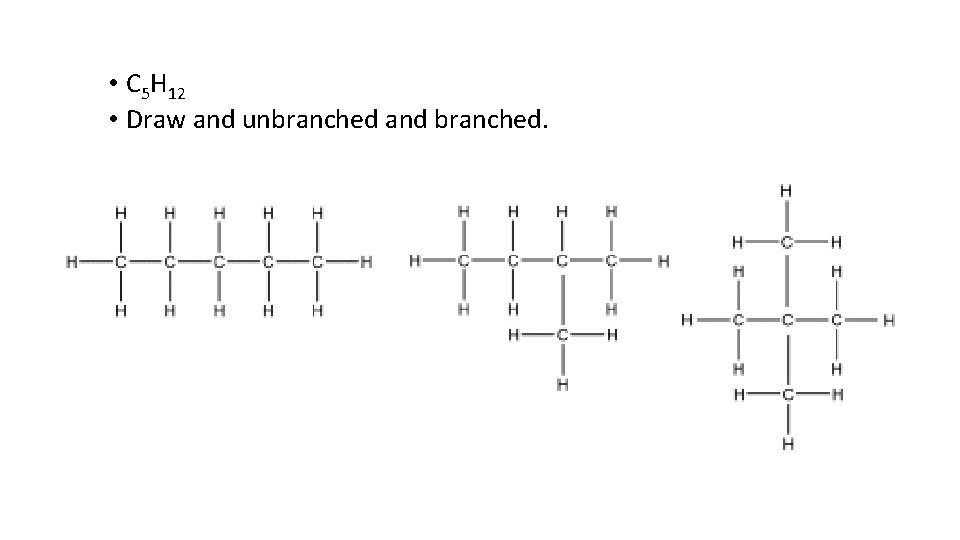
• C 5 H 12 • Draw and unbranched and branched.

• Naming branched alkanes • Determine the name of the parent chain • Count the longest continuous chain of carbon atoms – it may not appear to be in a straight line • Determine the prefix based on the number of carbons in the longest chain • Add the ending –ane.

• Determine the name of the branches • These names go in front of the parent chain. • Find the number of carbon that the branch is on, this needs to be the lowest numbered carbon atom. • Find the name of the group. • Find the root for the number of carbons in the chain (meth, prop, etc. ) • Add the suffix –yl • If there is more than one chain with the same number of carbons, use the following prefixes to indicate the number of that specific chain. • 2 = di • 3 = tri • 4 = tetra • 5 = penta • If more than one type of chain is present, list the chains in alphabetical order.
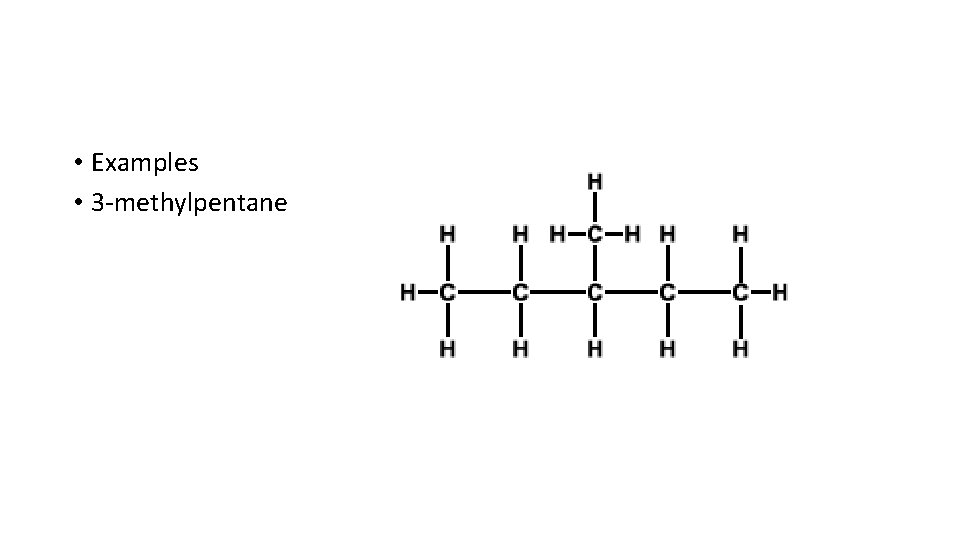
• Examples • 3 -methylpentane
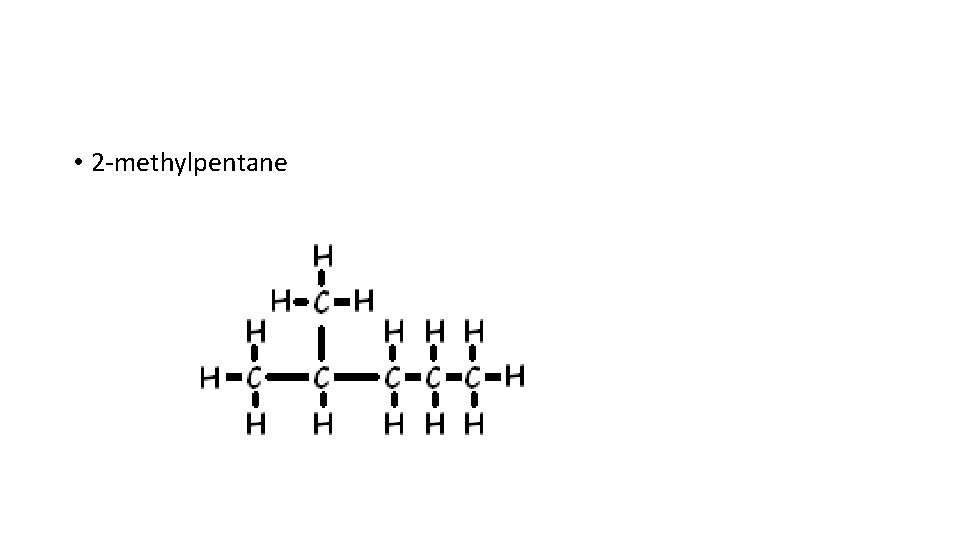
• 2 -methylpentane
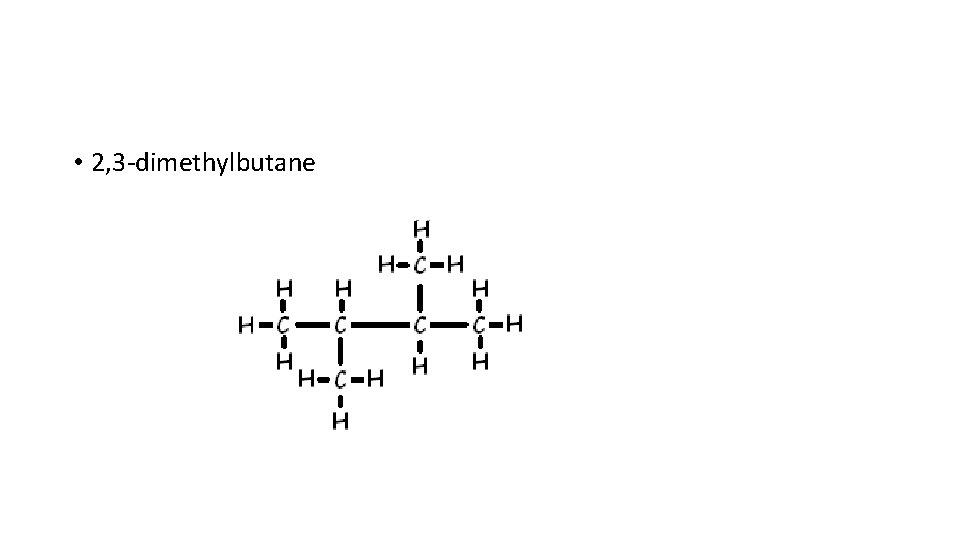
• 2, 3 -dimethylbutane

- Slides: 12