13 3 Factors affecting solubility 1 Nature of
13. 3 Factors affecting solubility 1. Nature of Solute/Solvent. - Like dissolves like (IMF) 2. Temperature i) Solids/Liquids- Solubility increases with Temperature Increase K. E. increases motion and collision between solute / solvent. ii) gas - Solubility decreases with Temperature Increase K. E. result in gas escaping to atmosphere. 3. Pressure i) Solids/Liquids - Very little effect Solids and Liquids are already close together, extra pressure will not increase solubility. ii) gas - Solubility increases with Pressure. Increase pressure squeezes gas solute into solvent.
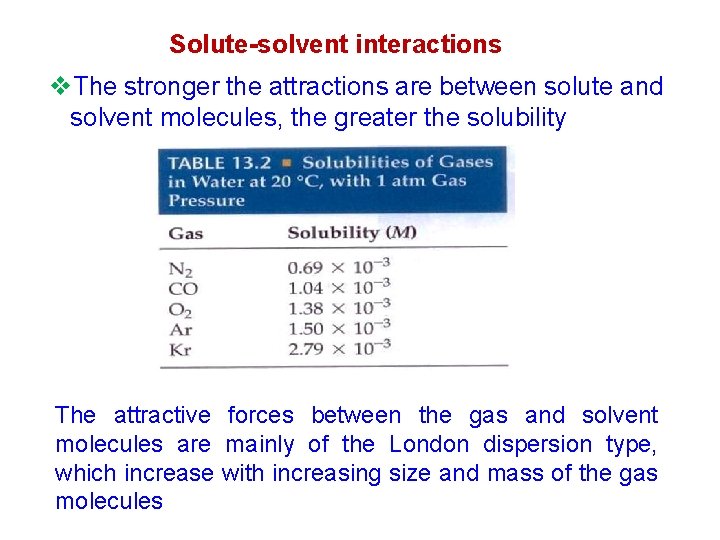
Solute-solvent interactions v. The stronger the attractions are between solute and solvent molecules, the greater the solubility The attractive forces between the gas and solvent molecules are mainly of the London dispersion type, which increase with increasing size and mass of the gas molecules

v. Polar liquids tend to dissolve readily in polar solvents v. Water is both polar and able to form hydrogen bonds, so polar molecules mix in all proportions with water v Nonpolar liquids tend to be insoluble in polar liquids. The attraction between the polar molecules and the nonpolar molecules is not sufficiently enough to allow the formation of a solution Hexane is insoluble in water (hexane is top)

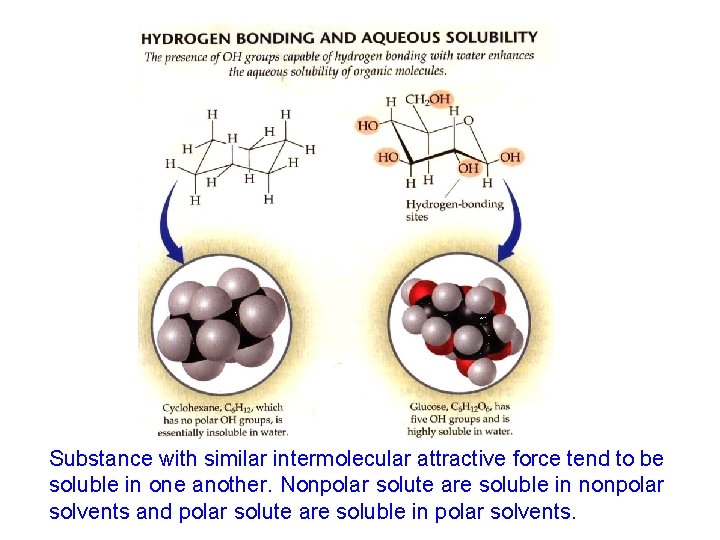
Substance with similar intermolecular attractive force tend to be soluble in one another. Nonpolar solute are soluble in nonpolar solvents and polar solute are soluble in polar solvents.

Example: Predict whether each of the following substances is more likely to dissolve in the nonpolar solvent carbon tetrachloride (CCl 4) or in water: C 7 H 16, Na 2 SO 4, HCl and I 2. Nonpolar solute are soluble in nonpolar solvents and polar solute are soluble in polar solvents. CCl 4 is nonpolar solvent H 2 O is polar solvent C 7 H 16 and I 2 are non polar solutes; more soluble in nonpolar solven, CCl 4 than polar water Na 2 SO 4 and HCl are polar solutes; more soluble in polar H 2 O than nonpolar CCl 4.

Pressure effects v. The solubilities of solids and liquids are not appreciably affected by pressure, whereas the solubility of a gas in any solvent is increased as the pressure over the solvent increases. At pressure of few atmosphere or less, solubility of gas solute follows Henry Law which states that the amount of solute gas dissolved in solution is directly proportional to the amount of pressure above the solution. Sg = k. Pg Sg, solubility of the gas in the solution phase (M, molarity) Pg, partial pressure of gas over the solution K, proportionality constant (Henry’s law constant)


Henry’s Law & Soft Drinks • Soft drinks contain “carbonated water” – water with dissolved carbon dioxide gas. • The drinks are bottled with a CO 2 pressure greater than 1 atm. • When the bottle is opened, the pressure of CO 2 decreases and the solubility of CO 2 also decreases, according to Henry’s Law. • Therefore, bubbles of CO 2 escape from solution.
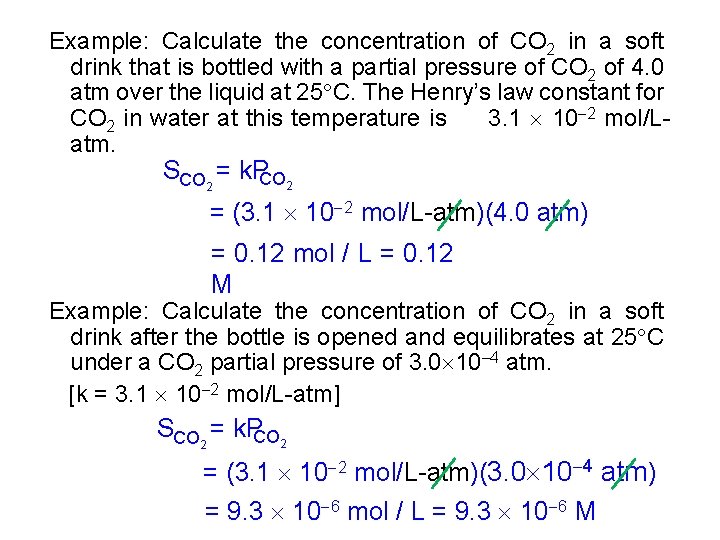
Example: Calculate the concentration of CO 2 in a soft drink that is bottled with a partial pressure of CO 2 of 4. 0 atm over the liquid at 25 C. The Henry’s law constant for CO 2 in water at this temperature is 3. 1 10 2 mol/Latm. SCO 2 = k. PCO 2 = (3. 1 10 2 mol/L-atm)(4. 0 atm) = 0. 12 mol / L = 0. 12 M Example: Calculate the concentration of CO 2 in a soft drink after the bottle is opened and equilibrates at 25 C under a CO 2 partial pressure of 3. 0 10 4 atm. [k = 3. 1 10 2 mol/L-atm] SCO 2 = k. PCO 2 = (3. 1 10 2 mol/L-atm)(3. 0 10 4 atm) = 9. 3 10 6 mol / L = 9. 3 10 6 M
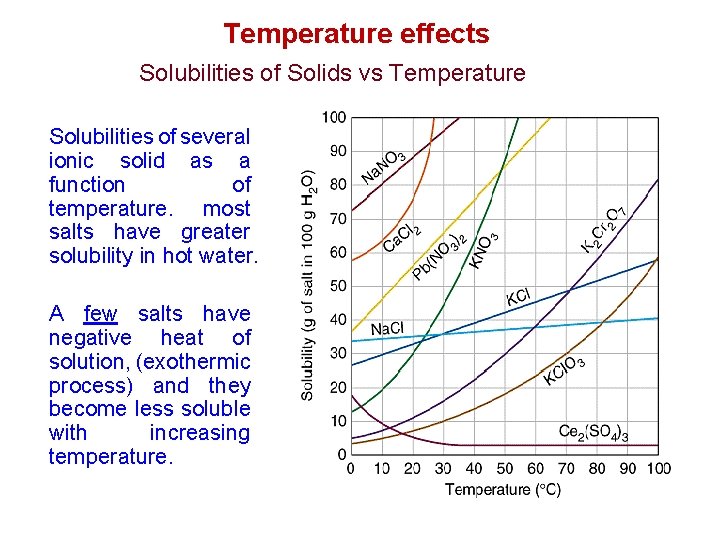
Temperature effects Solubilities of Solids vs Temperature Solubilities of several ionic solid as a function of temperature. most salts have greater solubility in hot water. A few salts have negative heat of solution, (exothermic process) and they become less soluble with increasing temperature.
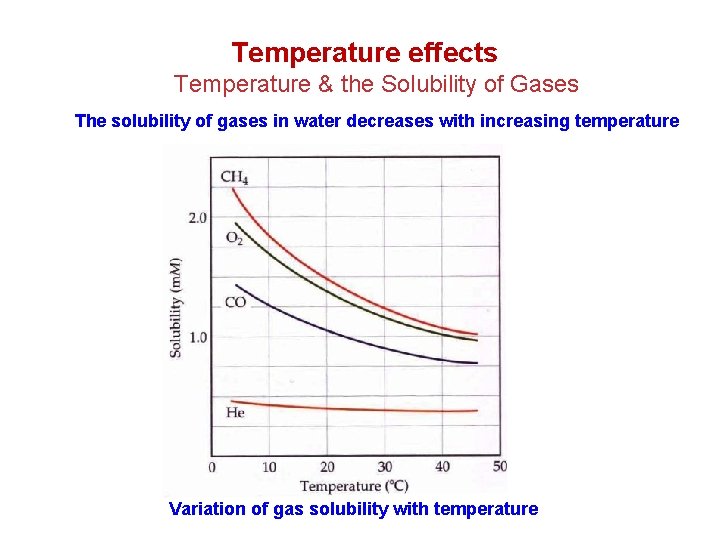
Temperature effects Temperature & the Solubility of Gases The solubility of gases in water decreases with increasing temperature Variation of gas solubility with temperature
- Slides: 12