13 1 Electrons and Chemical Bonds Valence Electrons

13. 1 Electrons and Chemical Bonds
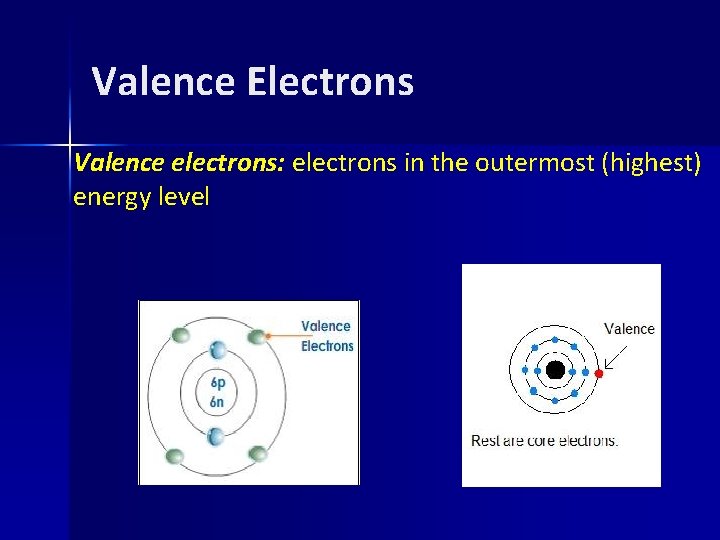
Valence Electrons Valence electrons: electrons in the outermost (highest) energy level
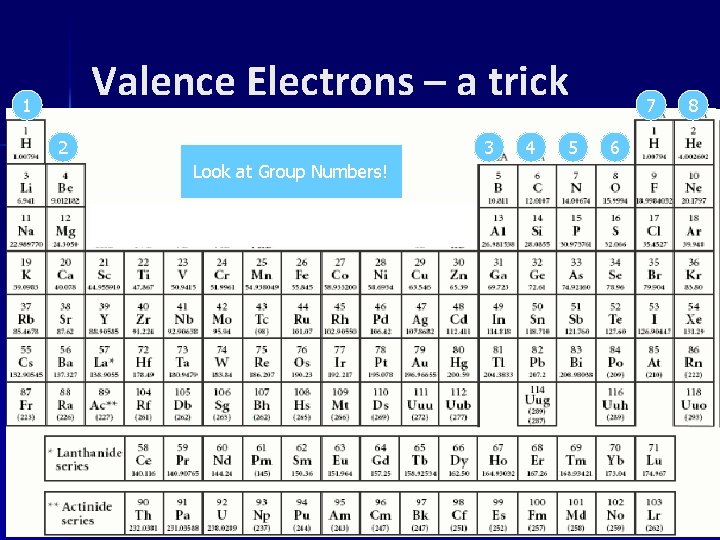
Valence Electrons – a trick 1 2 3 Look at Group Numbers! 4 5 7 6 8

How many Valence Electrons? Nitrogen (N) n Carbon (C) n Potassium (K) n

Lewis Dot Diagrams A Lewis dot diagram shows the element symbol surrounded by dots representing the valence electrons. Example: To draw a Lewis dot diagram: 1. Determine the number of valence electrons 2. Write the symbol and add dots for valence electrons
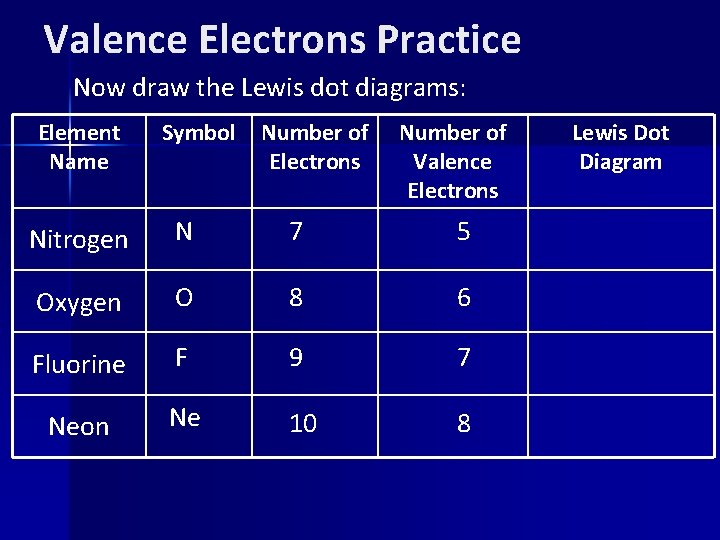
Valence Electrons Practice Now draw the Lewis dot diagrams: Element Name Symbol Number of Electrons Number of Valence Electrons Nitrogen N 7 5 Oxygen O 8 6 Fluorine F 9 7 Neon Ne 10 8 Lewis Dot Diagram
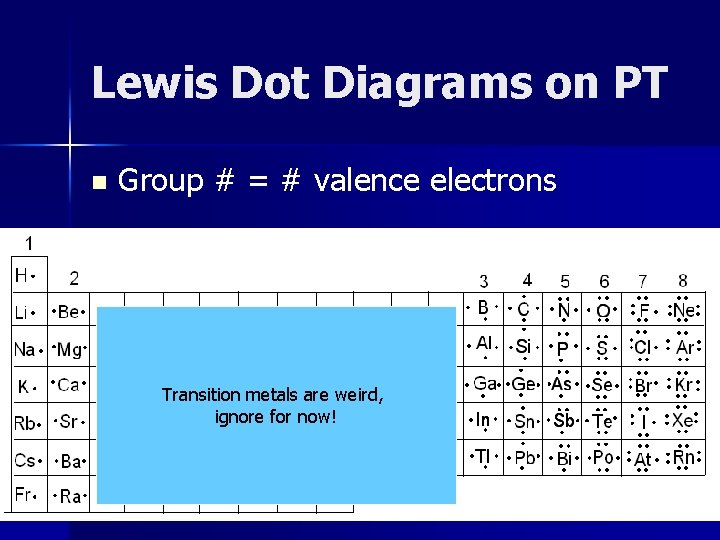
Lewis Dot Diagrams on PT n Group # = # valence electrons Transition metals are weird, ignore for now!
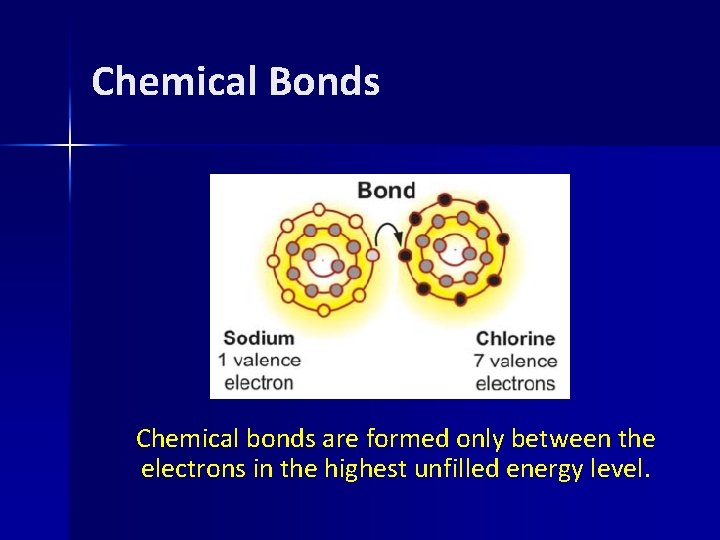
Chemical Bonds Chemical bonds are formed only between the electrons in the highest unfilled energy level.
Types of Chemical Bonds A chemical bond forms when atoms transfer or share electrons. Covalent Bonds – atoms share valence electrons Ionic Bonds – atoms transfer valence electrons

Dogs Teaching Chemistry! https: //www. youtube. com/watch? v=_M 9 khs 87 x. Q 8
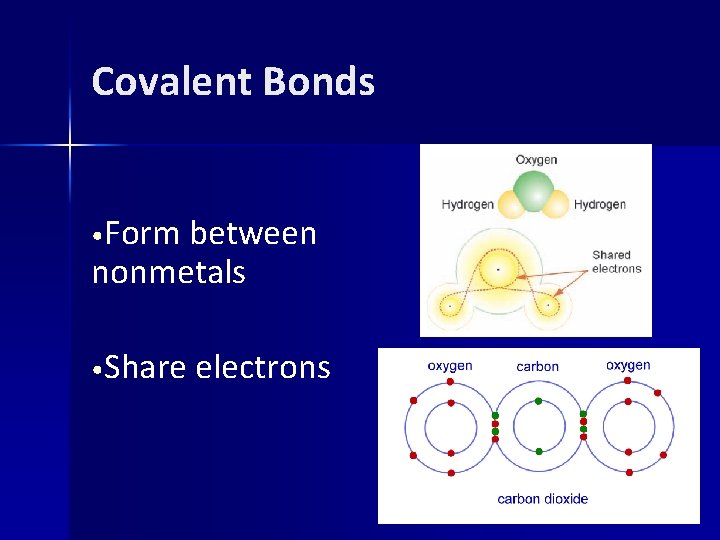
Covalent Bonds • Form between nonmetals • Share electrons
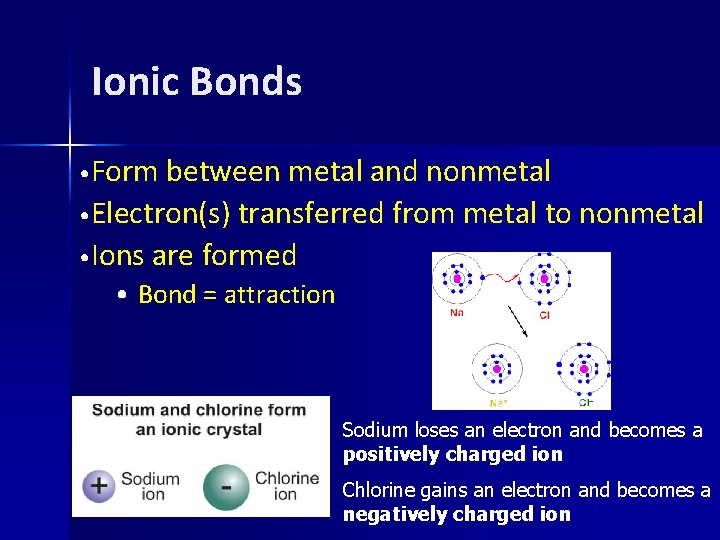
Ionic Bonds • Form between metal and nonmetal • Electron(s) transferred from metal to nonmetal • Ions are formed • Bond = attraction Sodium loses an electron and becomes a positively charged ion Chlorine gains an electron and becomes a negatively charged ion
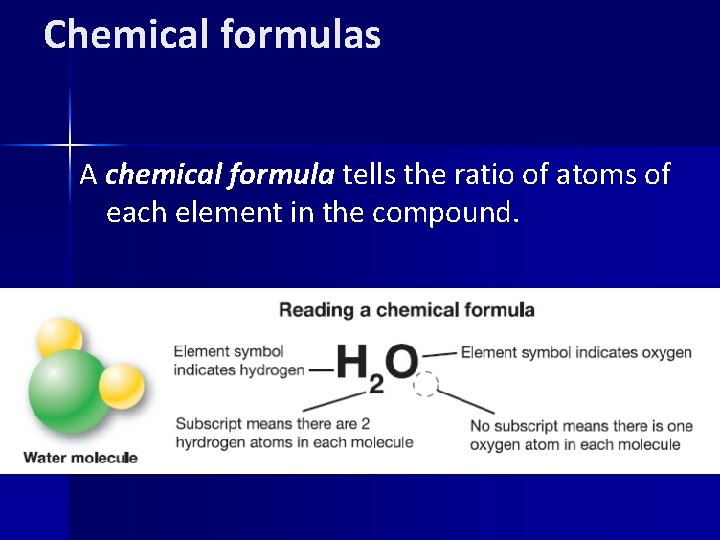
Chemical formulas A chemical formula tells the ratio of atoms of each element in the compound.

Lewis Dot Diagrams EXIT SLIP Answer the following questions about phosphorous: 1. 2. 3. How many total electrons does phosphorous have? How many valence electrons does phosphorous have? What is the Lewis dot diagram for phosphorous?
Groups… n n n n n Jean, Kalie, Chris – Lab station 1 Brianna, Jazz, Anna – Lab station 3 Jackie, Mandy, Randy – Lab station 5 Cole C, Tara, Dylan – Lab station 7 David, Sam, Maddie – Lab station 9 Devon, Amanda, Ayrias – Lab station 11 Cole F, Eli, Lysandra – Lab station 13 Juan, Andre, Gilberto – Desks Liz, Herlinda, Zach – Desks Mohamed, Tas, Avery – Desks
- Slides: 15