126 127 128 129 21 22 Pg 3
















- Slides: 23

1/26 & 1/27 1/28 & 1/29 2/1 & 2/2 Pg 3 Metal Alloys & Alchemy Lab Pg 3 Metal Alloys PT Trends Review PT Exam 2 Pg 4: Bonding Inquiry Experiment with metallic bonding Distinguish between alloys & elements Classify bond types based on metal & nonmetal description Find your testing number. Switch out calculators for your phones. Move bags to the back of the room. Exam is 40 minutes Sapling 1/27 PT test PT Test 2/2 & 2/3 Hmwk Finish Pg 4 Wk 20 Sapling due 2/3

PG 68 Pg 68 Header: I can distinguish between ionic, covalent, metallic bond properties Hmwk: Monoatomic ion quiz (lost your list? It’s online) Take notes over 1 of the videos online for homework

Get to know your team! • Each person draws a red interview card. Read your card to yourself. • You may ask 1 person at your table your interview question. • After they answer you must answer your own question too. • Each person at the table must answer at least 1 question • Rotate clockwise around the table

Bonding Inquiry • In your group sort the cards into 2 stacks • What are some differences between your groups?

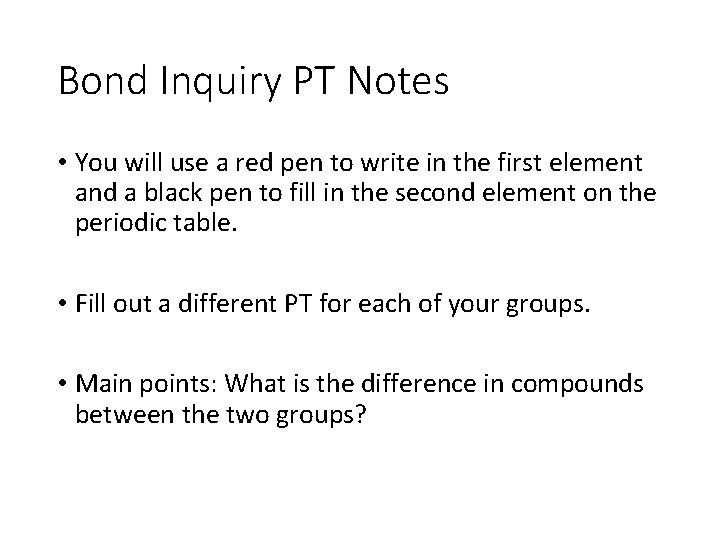
Bond Inquiry PT Notes • You will use a red pen to write in the first element and a black pen to fill in the second element on the periodic table. • Fill out a different PT for each of your groups. • Main points: What is the difference in compounds between the two groups?

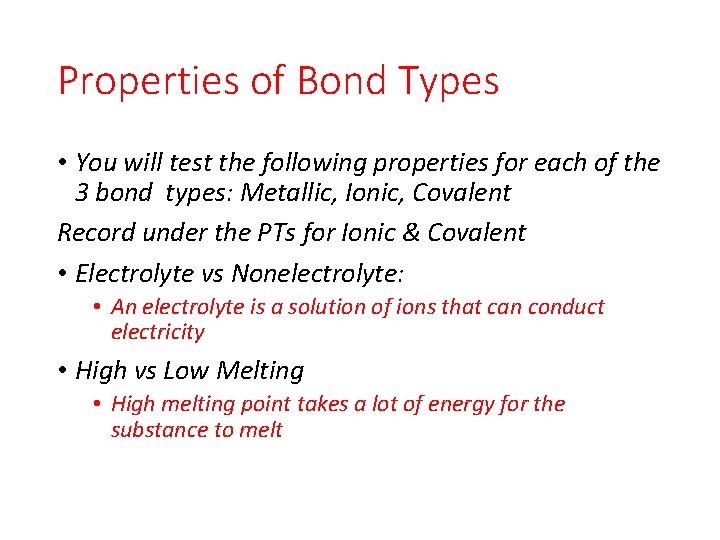
Properties of Bond Types • You will test the following properties for each of the 3 bond types: Metallic, Ionic, Covalent Record under the PTs for Ionic & Covalent • Electrolyte vs Nonelectrolyte: • An electrolyte is a solution of ions that can conduct electricity • High vs Low Melting • High melting point takes a lot of energy for the substance to melt

Bond Inquiry PT Notes • Ionic compounds contain metals + nonmetal • EX: Calcium Chloride Ca. Cl 2 • SUPER STRONG BONDS because of donated electrons! • Covalent compounds contain nonmetal + nonmetal • EX: Disulfur Pentoxide S 2 O 5 • Share electrons so everyone is happy!

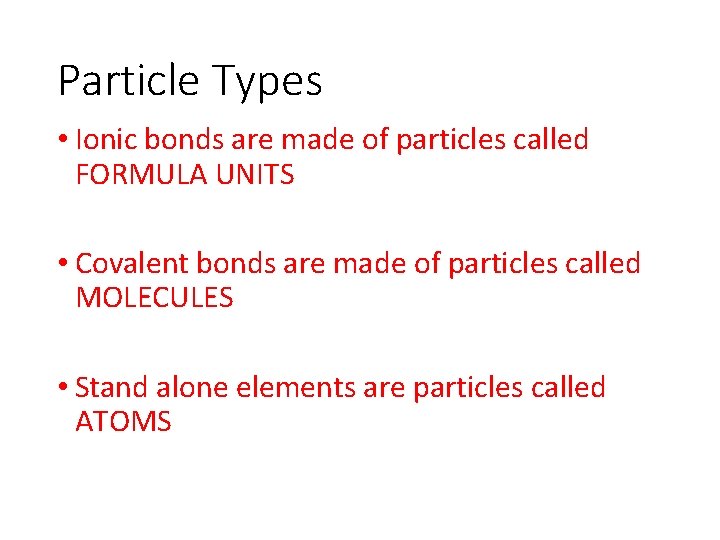
Particle Types • Ionic bonds are made of particles called FORMULA UNITS • Covalent bonds are made of particles called MOLECULES • Stand alone elements are particles called ATOMS

2 Step Metric Practice 1. A block of cheese won the county fair for weighing 546. 5 Mg how many mg is this? 2. If the mouse’s stomach can hold 8. 97 x 1010 μL of cheese, how many GL can it hold? 3. The block of cheese has a density of 2. 30 x 1014 miligrams per gigaliter. Can the mouse eat all the cheese?

Exit Ticket • Write your name • You need your yellow conversion chart! • How many Tm are in 3. 60 x 1021 nm?

PG 69 Pg 69 Header: I can write names of binary ionic compounds Hmwk: Ionic Naming Homework 1

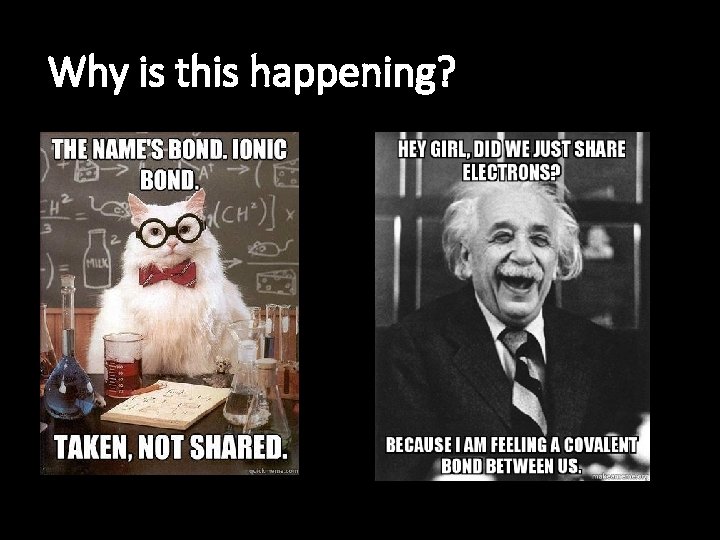
Why is this happening?

Draw a time when… 1. Opposites attracted • What’s common in your groups pictures? 2. Opposites took something from each other • What happened to the two things? 3. Opposites resolved conflict to make a strong bond • What did their resolution create?

Ionic Bead Activity- 35 mins • With your shoulder partner skim through pg 5 & 6 Discuss with your partner: • What do the circles represent? • Find 2 color pencils you will use for the entire activity.

• ALL BEADS must stay on the tray. If it hits the floor find it quick. If I find it before you get a zero on this! 1. Make sure you have all the cards listed on the tube & 8 beads for every card in your bag. 2. Move the beads! Notice patterns! Organize things how they make sense to you! 3. If you have doubles of an element you only need to draw it once. 4. When you finish with #4 raise your hand so I can check before you move on

Summarize the activity: With your partner answer the following: • Write a set of “rules” used for ionic bonding in the summary box • Review your naming rules. Summarize how to name an ionic compound • Now try to name all the compounds you created on pg 6

Ionic Naming Rules • In your groups summarize your video notes! • Always name the metal cation first • If it’s a transition/post-transition metal you must give it a roman numeral to tell it’s charge • I, III, IV, V, VII, VIII, IX, X • Name the nonmetal anion using the suffix –ide

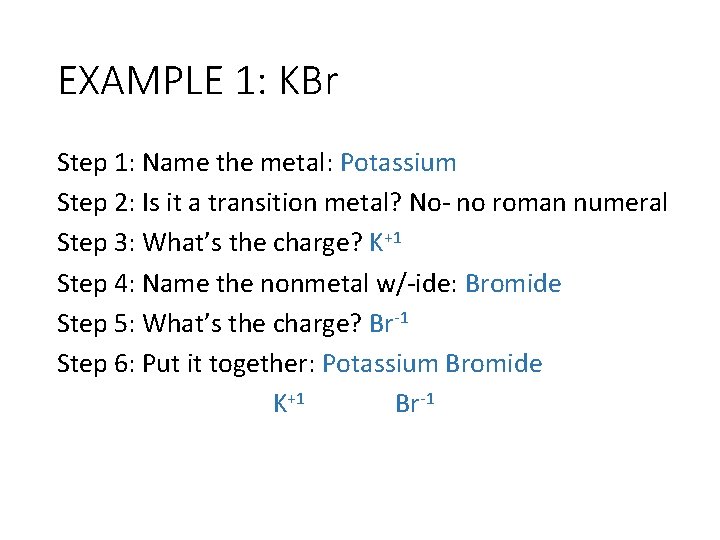
EXAMPLE 1: KBr Step 1: Name the metal: Potassium Step 2: Is it a transition metal? No- no roman numeral Step 3: What’s the charge? K+1 Step 4: Name the nonmetal w/-ide: Bromide Step 5: What’s the charge? Br-1 Step 6: Put it together: Potassium Bromide K+1 Br-1

EXAMPLE 1: Fe. O Step 1: Name the metal: Iron Step 2: Is it a transition metal? Yes- roman numeral Step 3: What’s the charge? Transition metals must equal & opposite the charge of the anion. O-2 charge so Fe+2 roman numeral (II) Step 4: Name the nonmetal w/-ide: Bromide Step 5: What’s the charge? Br-1 Put it together: Iron (II) Oxide Fe+2 O-2

We Do: LDS + Name • Ca. I 2 • Mg. Se • Ti. Cl 3

You Do: LDS + Name • K 3 P • Al. F 3 • Ni. Cl 2

EXCEPTIONS • There are 3 transition metals that do not receive roman numerals because they only have 1 set charge • Cd. Zn. Ag. Al 2213 • Cd+2 Zn+2 Ag+1 Al+3 Example: Ag. Br • Silver, Ag+1 charge, exception no roman numeral • Bromide, Br-1 charge • Silver Bromide

Exit Ticket: • Use the following list of elements to construct 3 LDS for the ionic bonds. • Rubidium + Oxygen • Barium + Chlorine • Tin with a +2 charge + Bromine • BONUS: Name the compounds you made. • Turn into white tray on your way out.