12202021 Compounds and Mixtures Elements 12202021 If a


- Slides: 12

12/20/2021 Compounds and Mixtures

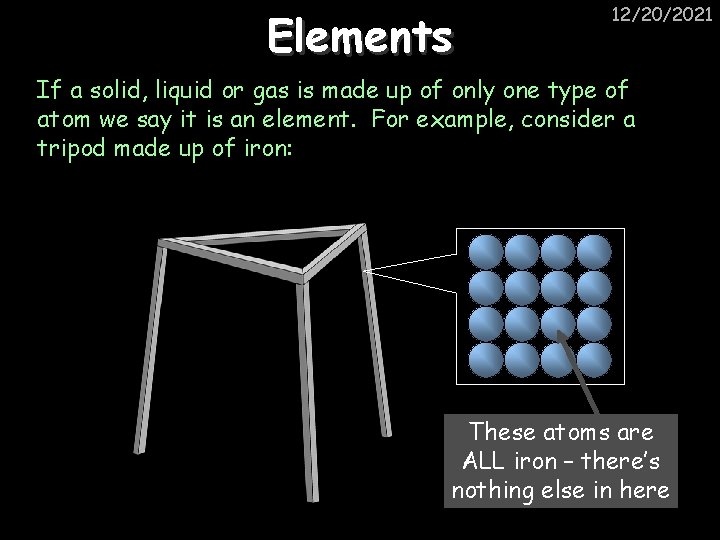
Elements 12/20/2021 If a solid, liquid or gas is made up of only one type of atom we say it is an element. For example, consider a tripod made up of iron: These atoms are ALL iron – there’s nothing else in here

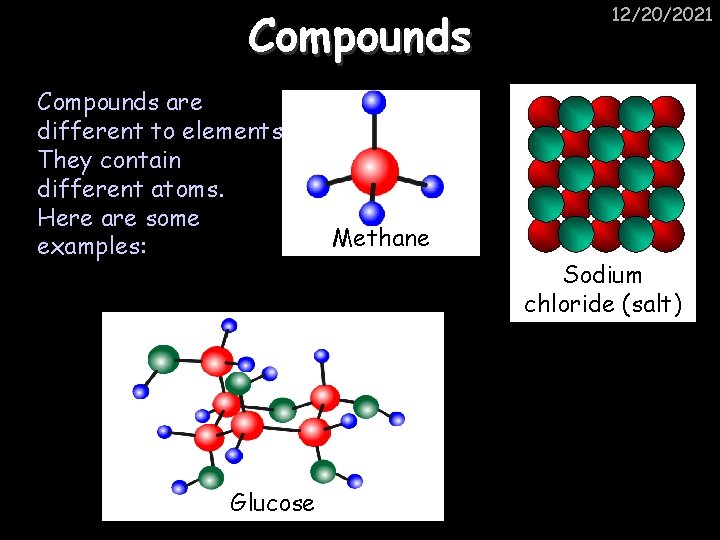
Compounds are different to elements. They contain different atoms. Here are some examples: Glucose 12/20/2021 Methane Sodium chloride (salt)

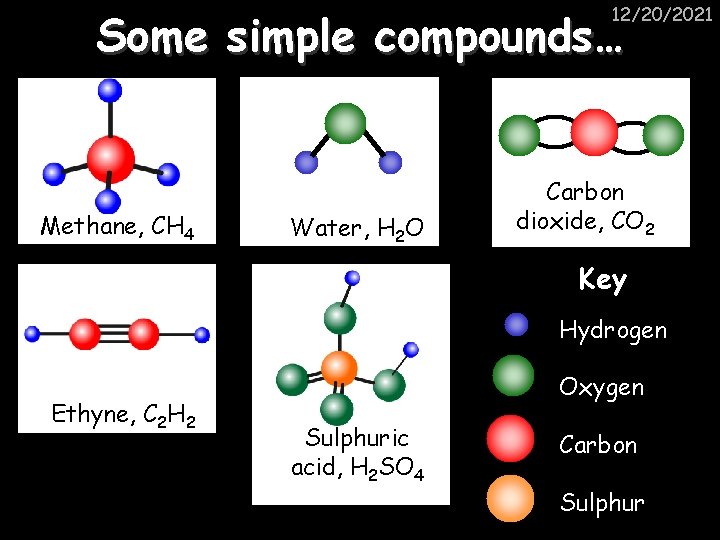
12/20/2021 Some simple compounds… Methane, CH 4 Water, H 2 O Carbon dioxide, CO 2 Key Hydrogen Ethyne, C 2 H 2 Oxygen Sulphuric acid, H 2 SO 4 Carbon Sulphur

Making compounds 12/20/2021 Compounds are made when two or more elements (or compounds) go through a chemical reaction. For example: 1) When carbon reacts with oxygen it usually forms _________ 2) When oxygen reacts with hydrogen it could form ______ 3) When iron reacts with oxygen it could form ______ There are usually “tell-tale” signs to show when a compound is being made, such as bubbling, heat given off, colour changes, fizzing etc. Water, iron oxide or carbon dioxide?

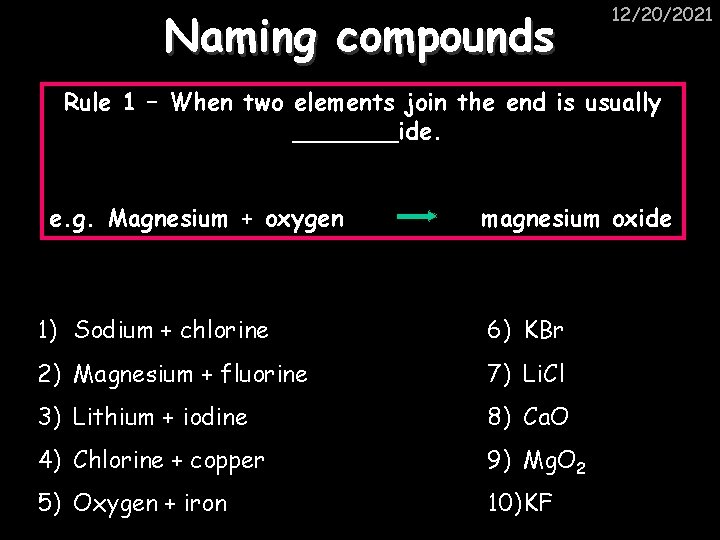
Naming compounds 12/20/2021 Rule 1 – When two elements join the end is usually _______ide. e. g. Magnesium + oxygen magnesium oxide 1) Sodium + chlorine 6) KBr 2) Magnesium + fluorine 7) Li. Cl 3) Lithium + iodine 8) Ca. O 4) Chlorine + copper 9) Mg. O 2 5) Oxygen + iron 10) KF

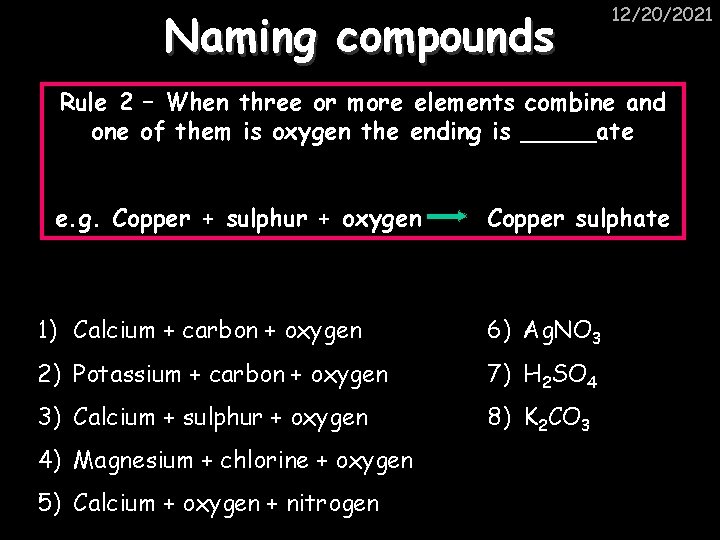
Naming compounds 12/20/2021 Rule 2 – When three or more elements combine and one of them is oxygen the ending is _____ate e. g. Copper + sulphur + oxygen Copper sulphate 1) Calcium + carbon + oxygen 6) Ag. NO 3 2) Potassium + carbon + oxygen 7) H 2 SO 4 3) Calcium + sulphur + oxygen 8) K 2 CO 3 4) Magnesium + chlorine + oxygen 5) Calcium + oxygen + nitrogen

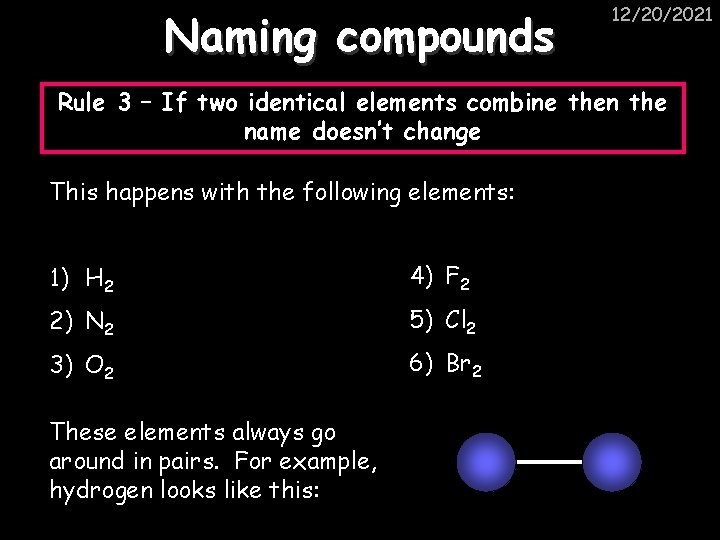
Naming compounds 12/20/2021 Rule 3 – If two identical elements combine then the name doesn’t change This happens with the following elements: 1) H 2 4) F 2 2) N 2 5) Cl 2 3) O 2 6) Br 2 These elements always go around in pairs. For example, hydrogen looks like this:

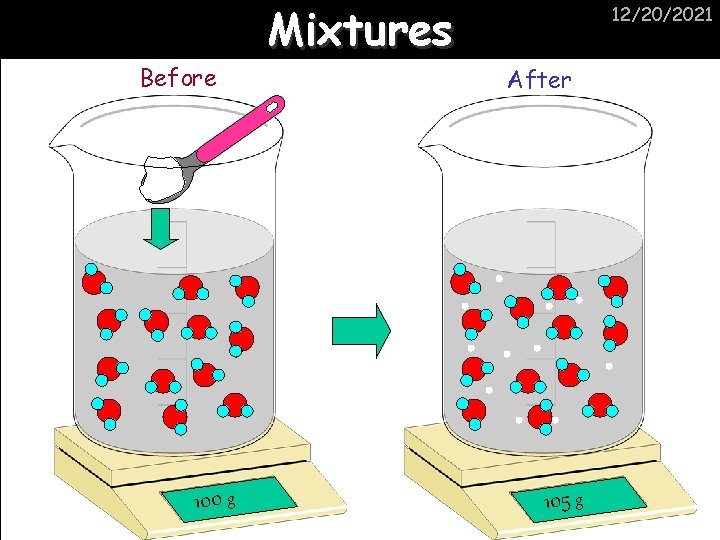
Before 100 g Mixtures 12/20/2021 After 105 g

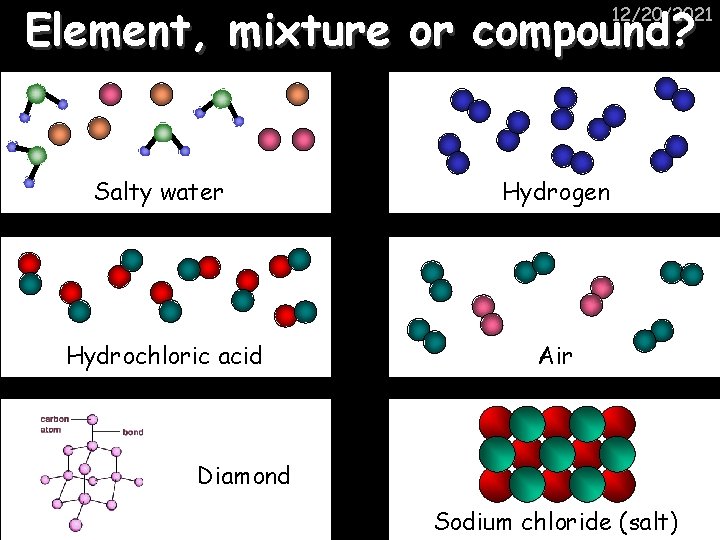
Element, mixture or compound? 12/20/2021 Salty water Hydrogen Hydrochloric acid Air Diamond Sodium chloride (salt)

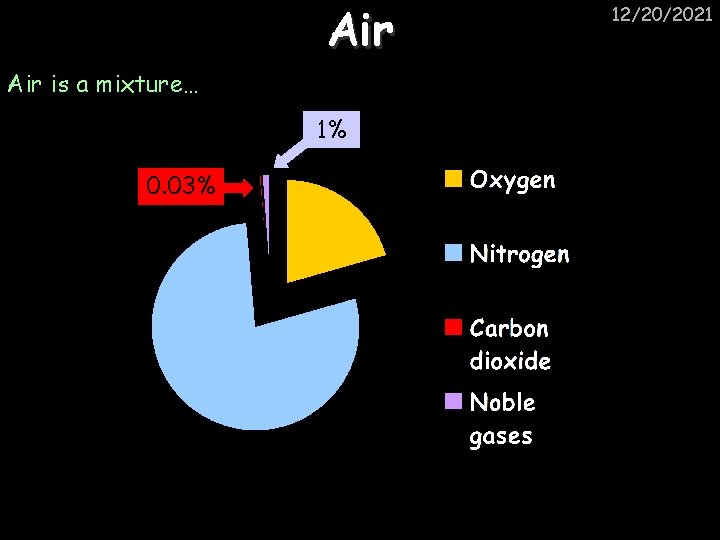
Air 12/20/2021 Air is a mixture… 1% 21% 0. 03% 79% 18% 79%

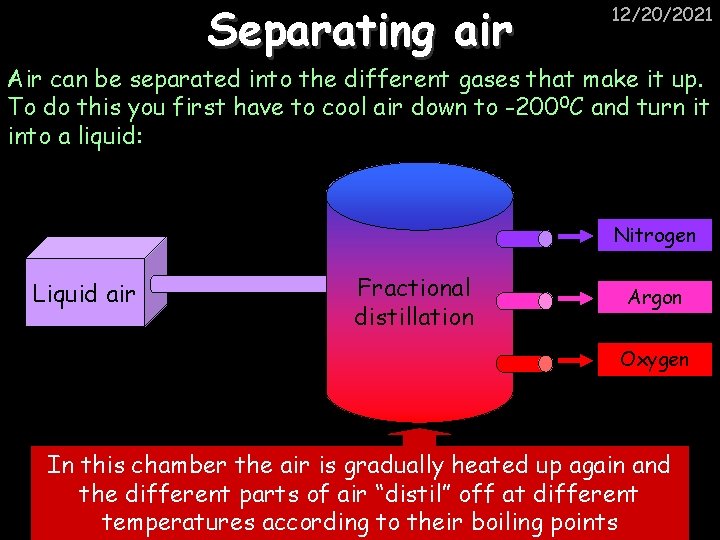
Separating air 12/20/2021 Air can be separated into the different gases that make it up. To do this you first have to cool air down to -200 0 C and turn it into a liquid: Nitrogen Liquid air Fractional distillation Argon Oxygen In this chamber the air is gradually heated up again and the different parts of air “distil” off at different temperatures according to their boiling points