12122021 Starter Activity Draw an ionic bonding diagram
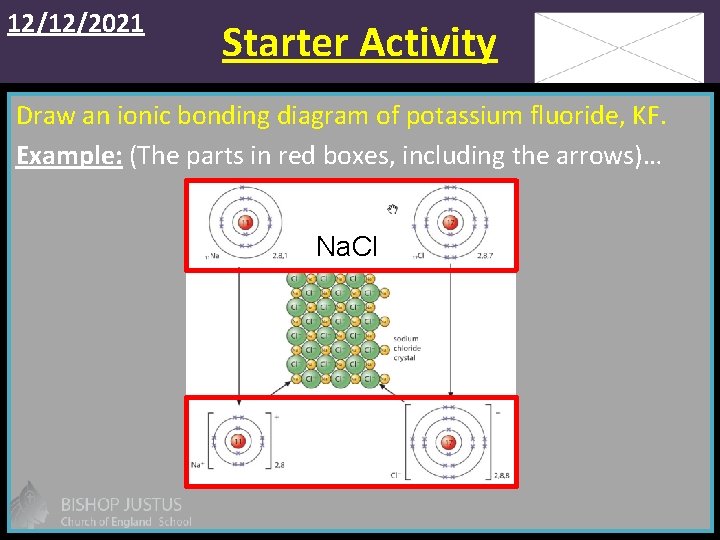
12/12/2021 Starter Activity Draw an ionic bonding diagram of potassium fluoride, KF. Example: (The parts in red boxes, including the arrows)… Na. Cl
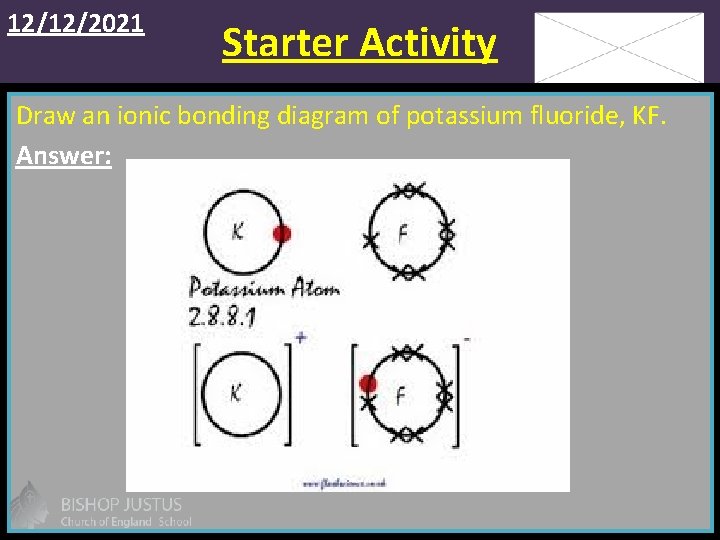
12/12/2021 Starter Activity Draw an ionic bonding diagram of potassium fluoride, KF. Answer:

12/12/2021 Learning Outcomes: Covalent bonding • A*/A: Understand that some elements form molecules on their own, but most molecules are compound • A/B: Identify the bonds formed between atoms • B/C: Explain how non metals form molecules. Key vocabulary: Compounds, Elements, covalent bonding
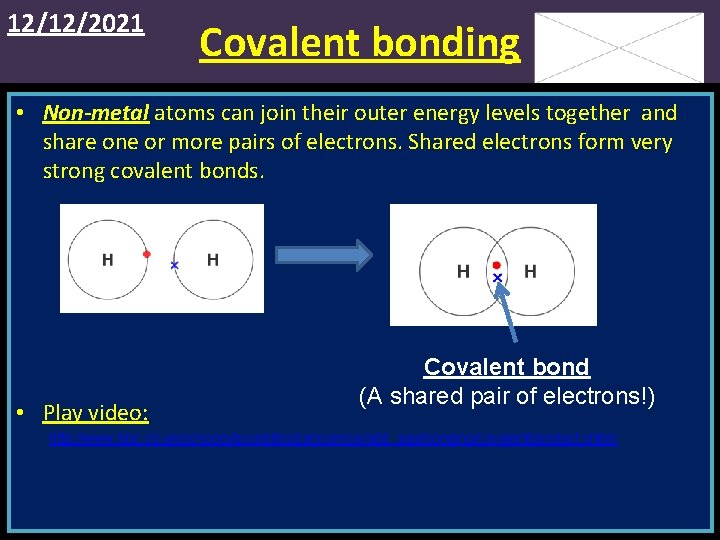
12/12/2021 Covalent bonding • Non-metal atoms can join their outer energy levels together and share one or more pairs of electrons. Shared electrons form very strong covalent bonds. • Play video: Covalent bond (A shared pair of electrons!) http: //www. bbc. co. uk/schools/gcsebitesize/science/add_aqa/bonding/covalentbondact. shtml
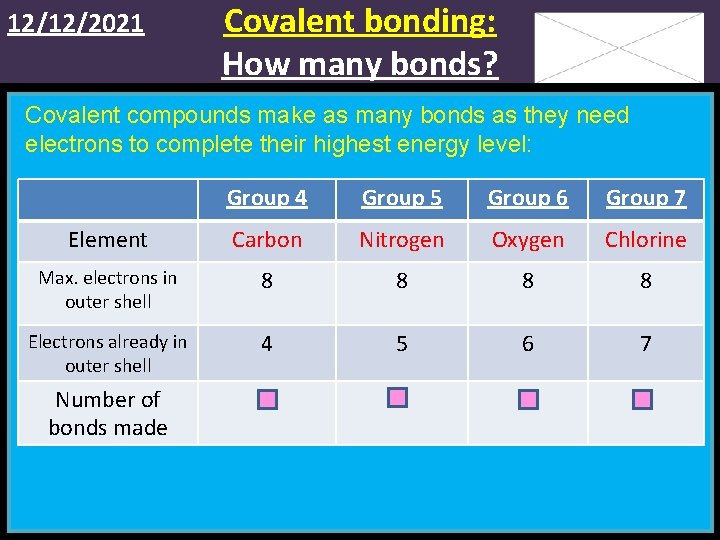
12/12/2021 Covalent bonding: How many bonds? Covalent compounds make as many bonds as they need electrons to complete their highest energy level: Group 4 Group 5 Group 6 Group 7 Element Carbon Nitrogen Oxygen Chlorine Max. electrons in outer shell 8 8 Electrons already in outer shell 4 5 6 7 Number of bonds made 4 3 2 1
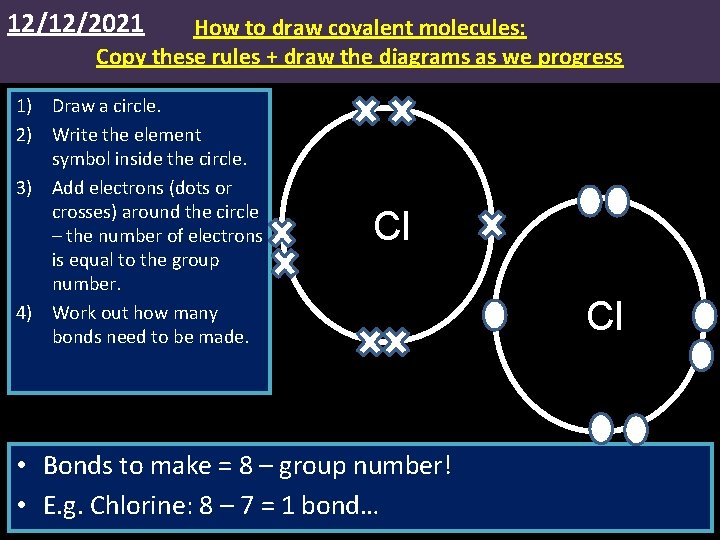
12/12/2021 How to draw covalent molecules: Copy these rules + draw the diagrams as we progress 1) Draw a circle. 2) Write the element symbol inside the circle. 3) Add electrons (dots or crosses) around the circle – the number of electrons is equal to the group number. 4) Work out how many bonds need to be made. Cl • Bonds to make = 8 – group number! • E. g. Chlorine: 8 – 7 = 1 bond… Cl
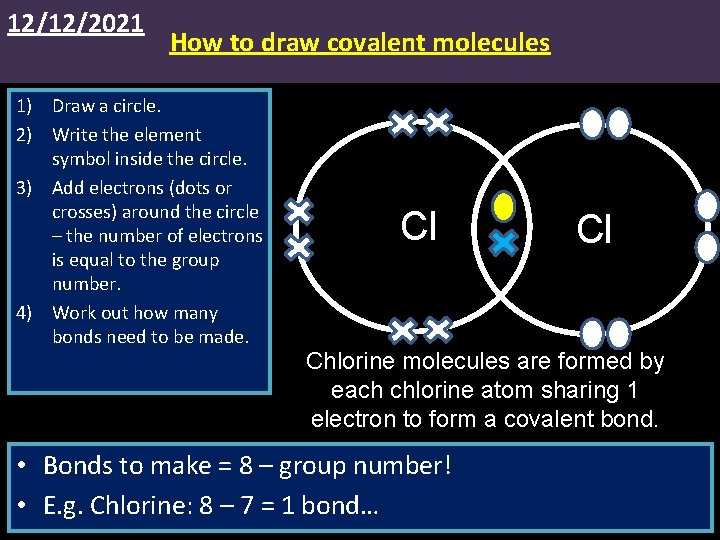
12/12/2021 How to draw covalent molecules 1) Draw a circle. 2) Write the element symbol inside the circle. 3) Add electrons (dots or crosses) around the circle – the number of electrons is equal to the group number. 4) Work out how many bonds need to be made. Cl Cl Chlorine molecules are formed by each chlorine atom sharing 1 electron to form a covalent bond. • Bonds to make = 8 – group number! • E. g. Chlorine: 8 – 7 = 1 bond…
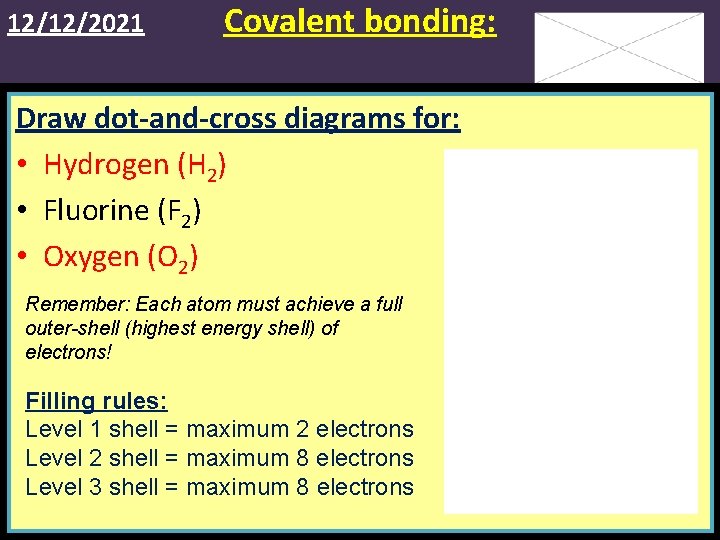
12/12/2021 Covalent bonding: Draw dot-and-cross diagrams for: • Hydrogen (H 2) • Fluorine (F 2) • Oxygen (O 2) Remember: Each atom must achieve a full outer-shell (highest energy shell) of electrons! Filling rules: Level 1 shell = maximum 2 electrons Level 2 shell = maximum 8 electrons Level 3 shell = maximum 8 electrons
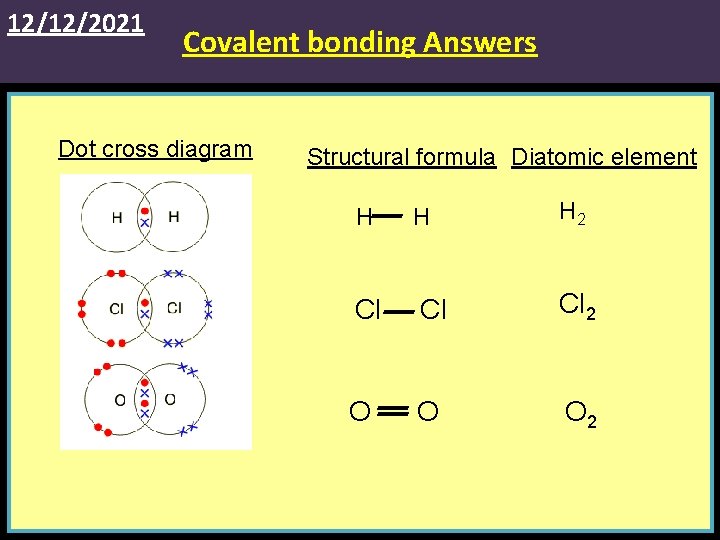
12/12/2021 Covalent bonding Answers Dot cross diagram Structural formula Diatomic element H H H 2 Cl Cl Cl 2 O O O 2
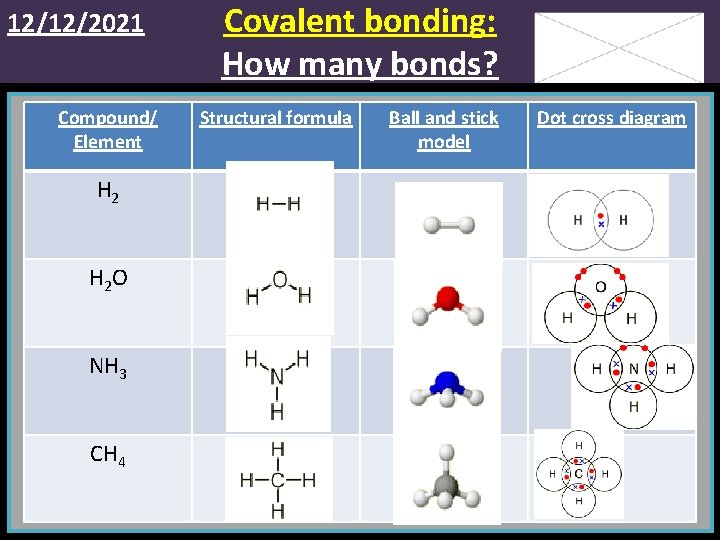
12/12/2021 Compound/ Element H 2 O NH 3 CH 4 Covalent bonding: How many bonds? Structural formula Ball and stick model Dot cross diagram
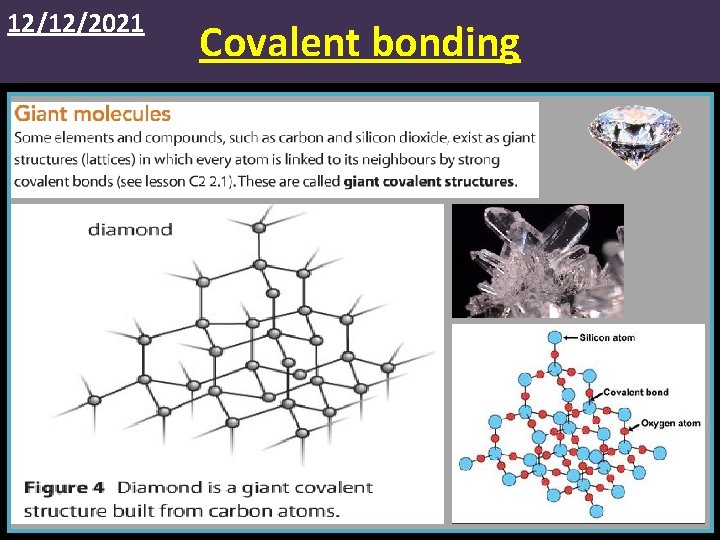
12/12/2021 Covalent bonding
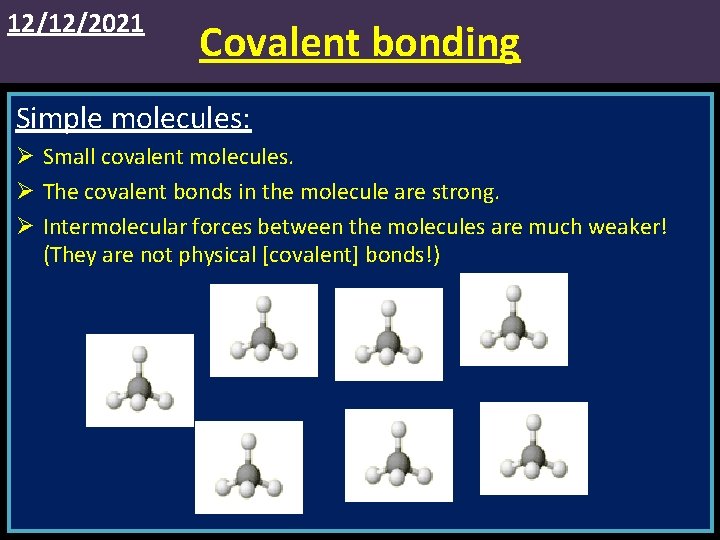
12/12/2021 Covalent bonding Simple molecules: Ø Small covalent molecules. Ø The covalent bonds in the molecule are strong. Ø Intermolecular forces between the molecules are much weaker! (They are not physical [covalent] bonds!)

12/12/2021 Covalent bonding Answer these!

12/12/2021 Learning Outcomes: Covalent bonding • A*/A: Understand that some elements form molecules on their own, but most moleules are compound • A/B: Identify the bonds formed between atoms • B/C: Explain how non metals form molecules. Key vocabulary: Compounds, Elements, covalent bonding
- Slides: 14