12 Structure Determination Mass Spectrometry 12 1 Mass


- Slides: 16

12. Structure Determination: Mass Spectrometry

12. 1 Mass Spectrometry (MS), Mass Spectrum n n n Sample vaporized and bombarded by energetic electrons that remove an electron, creating a cation-radical. A radical cation carries a positive charge and has an unpaired electron Bonds in cation radicals begin to break (fragment) The fragmentation pattern depends upon the class of compounds. The fragmentation processes favored are the ones that form the most stable cations Plot mass of ions (m/z) (x-axis) versus the intensity of the signal (corresponding to the number of ions) (y-axis) 2

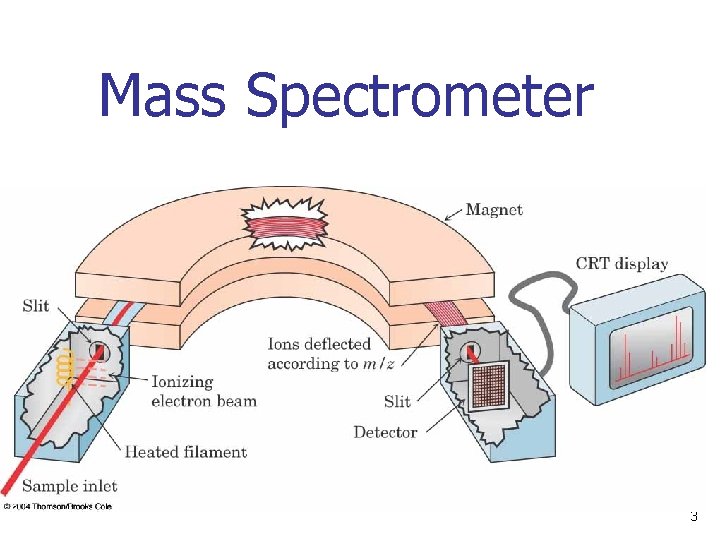
Mass Spectrometer 3

The Mass Spectrum n n n Tallest peak is base peak (100%). Other peaks listed as the % of that peak Peak that corresponds to the unfragmented radical cation is parent peak or molecular ion (M+): usually correspond to the highest mass in the spectrum The neutral fragments that may be lost in a fragmentation do not appear in the mass spectrum but can be accounted for by differences in masses between a peak and the molecular ion peak 4

Alkanes a. n-alkanes • For straight-chain alkanes, a molecular ion peak can be observed. • Fragmentation occurs by breaking of Carbon bonds. • As the chain length of an n-alkane increases, the chance of finding the fragment corresponding to the loss of a CH 3 unit decreases. • Alkanes fragment to form clusters of peaks which are separated from the neighboring clusters by 14 mass units accounted for by a CH 2. • Within a cluster, additional peaks are accounted for by losses of 1 or 2 H atoms b. Branched alkanes • The more branched the alkane, the lower the intensity of the molecular ion peak. May be even absent • Secondary and tertiary carbocation fragments 5

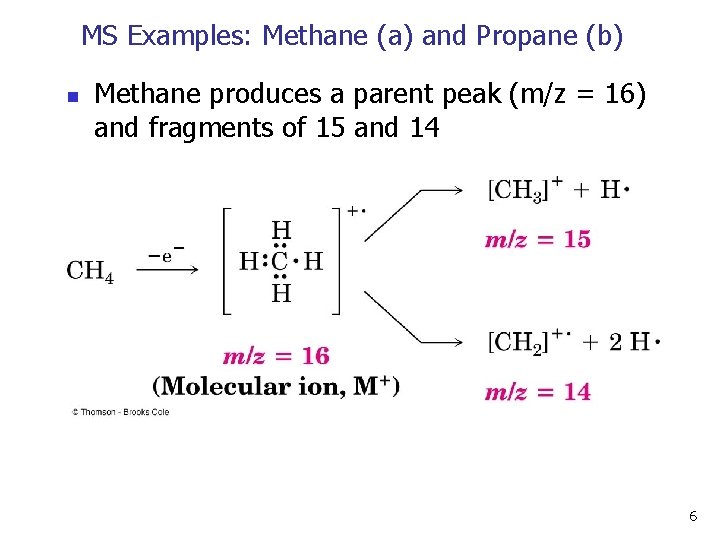
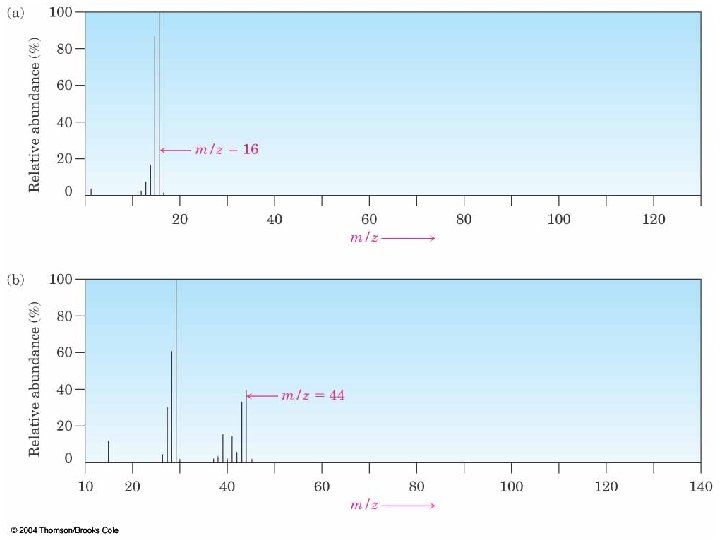
MS Examples: Methane (a) and Propane (b) n Methane produces a parent peak (m/z = 16) and fragments of 15 and 14 6

7

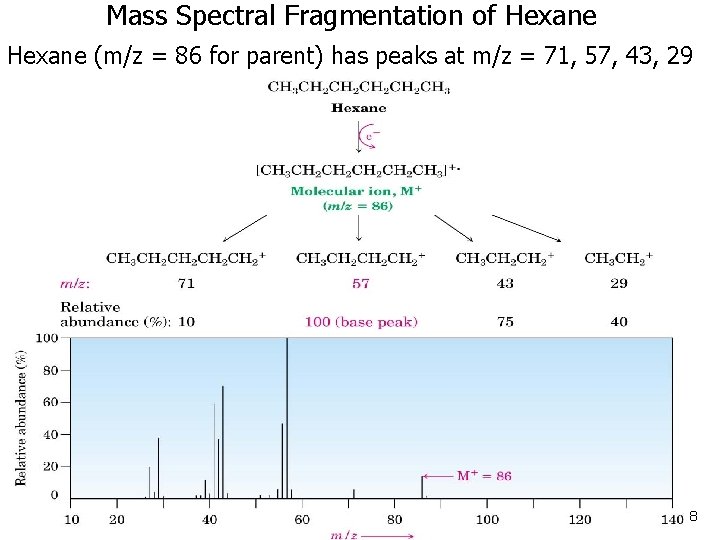
Mass Spectral Fragmentation of Hexane (m/z = 86 for parent) has peaks at m/z = 71, 57, 43, 29 8

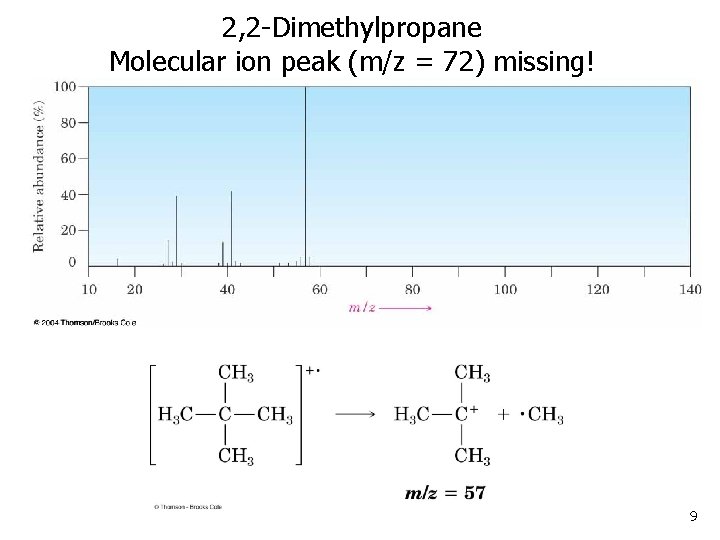
2, 2 -Dimethylpropane Molecular ion peak (m/z = 72) missing! 9

Cycloalkanes • Relatively intense molecular peak present • Cleavage of two Carbon bonds required for fragmentation • The common fragmentation involves loss of a molecule of ethene either from the parent molecule or from an intermediate radical ion. • In substituted cycloalkanes, a common fragmentation involves loss of the substituent. 10

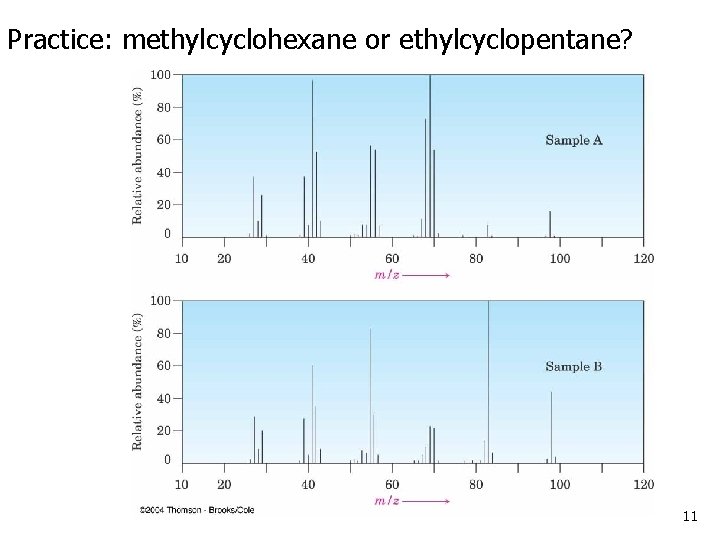
Practice: methylcyclohexane or ethylcyclopentane? 11

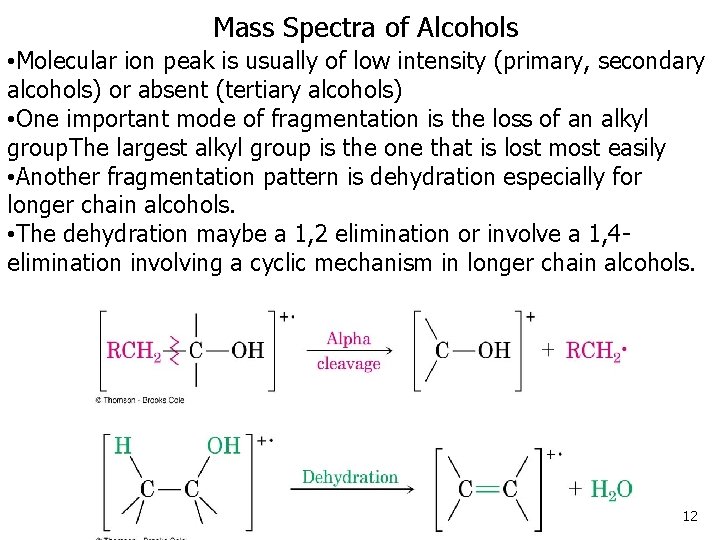
Mass Spectra of Alcohols • Molecular ion peak is usually of low intensity (primary, secondary alcohols) or absent (tertiary alcohols) • One important mode of fragmentation is the loss of an alkyl group. The largest alkyl group is the one that is lost most easily • Another fragmentation pattern is dehydration especially for longer chain alcohols. • The dehydration maybe a 1, 2 elimination or involve a 1, 4 elimination involving a cyclic mechanism in longer chain alcohols. 12

12. 2 Interpreting Mass Spectra n n Molecular weight from the mass of the molecular ion Double-focusing instruments provide highresolution “exact mass” n n 0. 0001 atomic mass units – distinguishing specific atoms Example MW “ 72” is ambiguous: C 5 H 12 and C 4 H 8 O but: n n C 5 H 12 72. 0939 amu exact mass C 4 H 8 O 72. 0575 amu exact mass Result from fractional mass differences of atoms 16 O = 15. 99491, 12 C = 12. 0000, 1 H = 1. 00783 13

Use of Heavier Isotope Peaks n Peaks above the molecular weight appear as a result of naturally occurring heavier isotopes in the sample + n (M+1) peak: one mass unit heavier than the M peak. + n (M+2) peak: two mass units heavier than the M peak. + n If 1 Br is present in the formula, M+2 peak as large as M peak n If 1 Cl is present in the formula, M+2 peak a third as large as M+ peak + n A m/z peak at 127 due to I n If 1 S is present in the formula, intensity of M+2 = 4% of that of M+ peak + n If 1 N is present in the formula, odd M peak 14

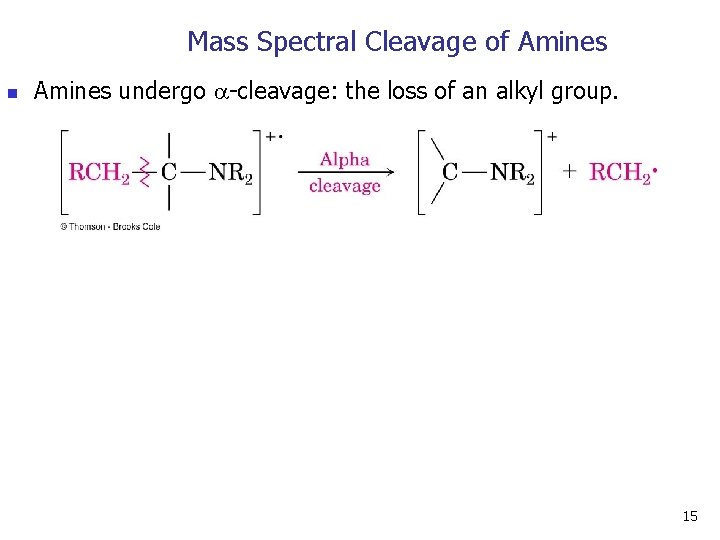
Mass Spectral Cleavage of Amines n Amines undergo -cleavage: the loss of an alkyl group. 15

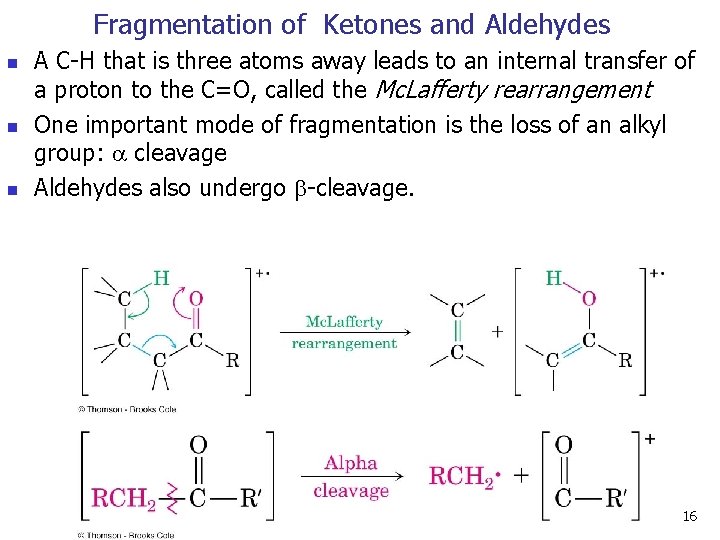
Fragmentation of Ketones and Aldehydes n n n A C-H that is three atoms away leads to an internal transfer of a proton to the C=O, called the Mc. Lafferty rearrangement One important mode of fragmentation is the loss of an alkyl group: cleavage Aldehydes also undergo -cleavage. 16