12 March 2021 Todays Title CW Isotops Learning








- Slides: 17

12 March 2021 Today’s Title: CW: Isotops Learning Question: What is an isotope ? Starter – http: //www. bbc. co. uk/learningzone/clips/ato ms-and-isotopes/10662. html

Key words… • Atom • Proton • Electron • Neutron • Mass number • Atomic number

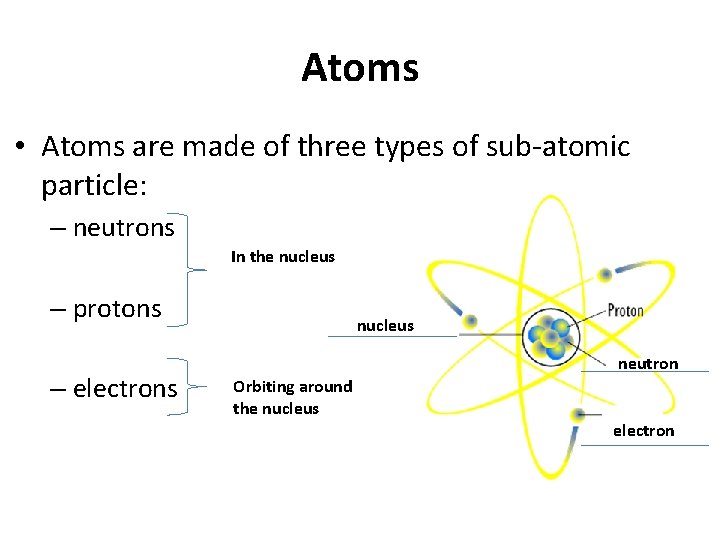
Atoms • Atoms are made of three types of sub-atomic particle: – neutrons In the nucleus – protons – electrons nucleus neutron Orbiting around the nucleus electron

Atoms • Atoms are made up of two main parts, the nucleus and orbiting electrons. • Electrons can be lost or gained and this forms charged particles - ions • The nucleus contains two types of particles called protons and neutrons • As protons, neutrons and electrons are the building blocks of atoms they're called sub-atomic particles

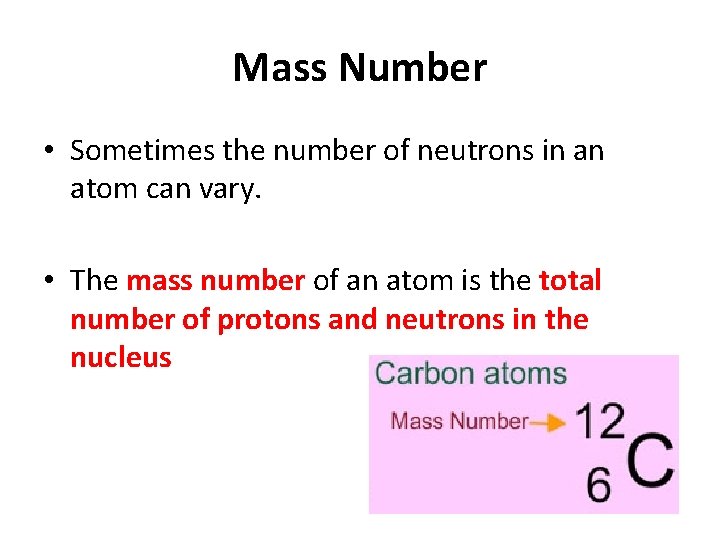
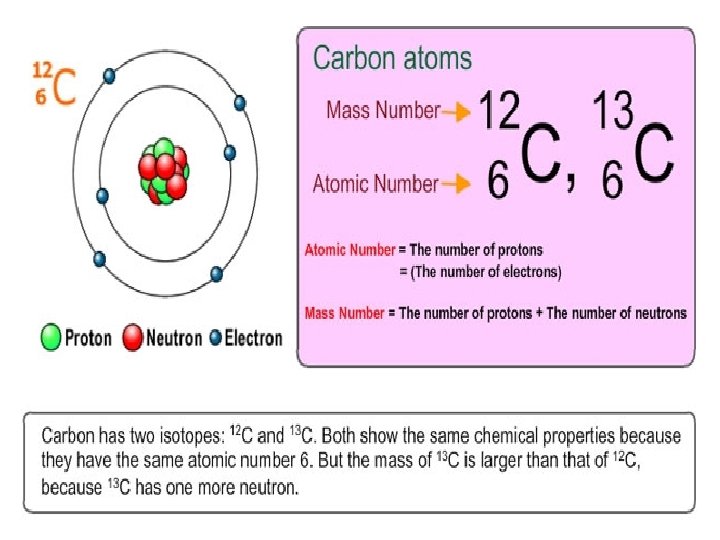
Mass Number • Sometimes the number of neutrons in an atom can vary. • The mass number of an atom is the total number of protons and neutrons in the nucleus

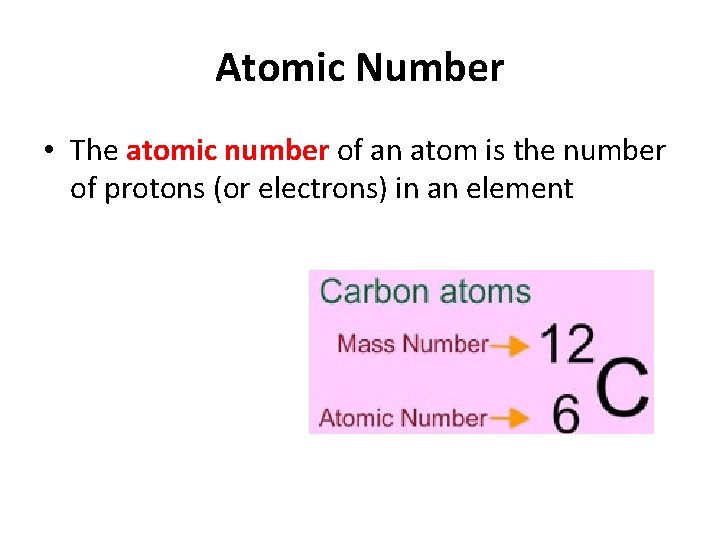
Atomic Number • The atomic number of an atom is the number of protons (or electrons) in an element

Quick questions 1. What does the atomic number of an element tell you? 2. What does the mass number of an element tell you?

1 What does the atomic number of an element tell you? A: The number of protons in the nucleus. 2 What does the mass number of an element tell you? A: The total number of protons and neutrons.

Isotopes • Two atoms of the same element will always have the same atomic number • The mass numbers can change if they contain different numbers of neutrons • Atoms of a single element that have different numbers of neutrons are called isotopes


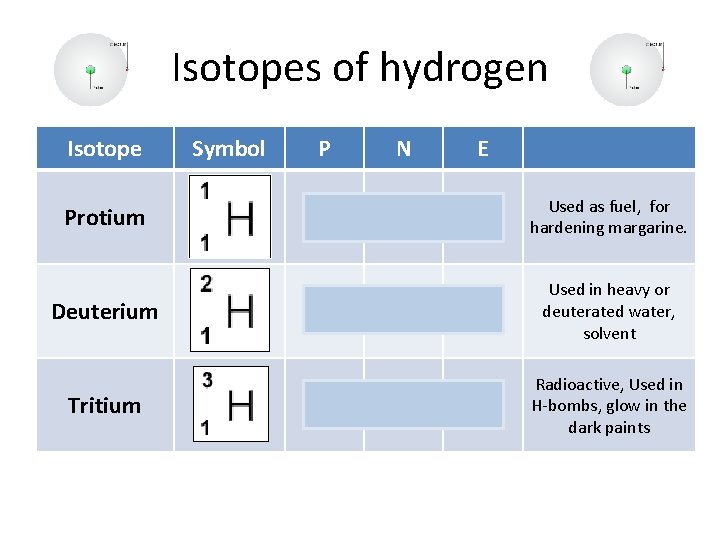
Isotopes of hydrogen Isotope Protium Deuterium Tritium Symbol P N E 1 0 1 Used as fuel, for hardening margarine. 1 Used in heavy or deuterated water, solvent 1 Radioactive, Used in H-bombs, glow in the dark paints 1 1 1 2

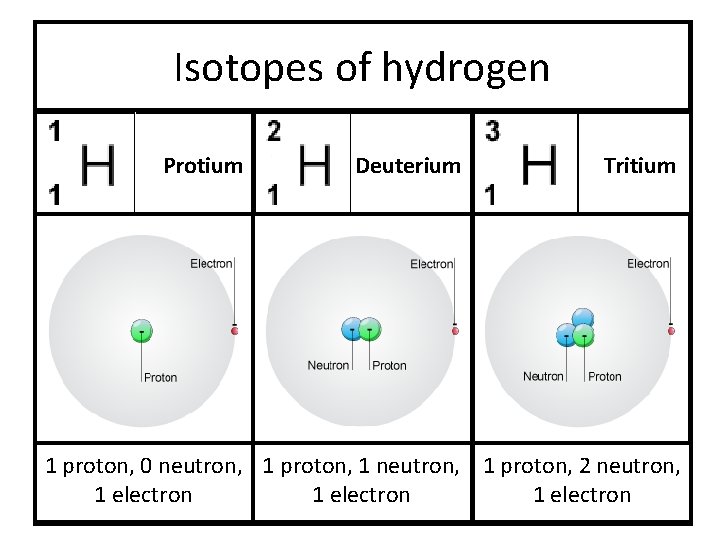
Isotopes of hydrogen Protium Deuterium Tritium 1 proton, 0 neutron, 1 proton, 1 neutron, 1 proton, 2 neutron, 1 electron

Quick questions 3. A helium nucleus has 2 protons and 2 neutrons. Write down the symbol for the helium nucleus, showing the atomic number and mass number 4. The symbol for a beryllium nucleus is Be. How many of the following particles does it contain? a. Protons b. neutrons

Your task • Complete questions 5 -8 from page 253 of your textbook.

3 A helium nucleus has 2 protons and 2 neutrons. Write down the symbol for the helium nucleus, showing the atomic number and mass number. A: 42 He. 4 The symbol for a beryllium nucleus is 94 Be. How many of the following particles does it contain? a protons A: 4 protons b neutrons. A: 5 neutrons

5. A: a 4, 5, 4 b Beryllium, boron, beryllium c 10 4 Be, 10 9 5 B, 4 Be 6 An atom of fluorine has 9 protons and 10 neutrons. Write down the symbol for a fluorine nucleus, showing the atomic number and nucleon number. A: 19 9 F 7 Sam says that as two different atoms have the same mass number, they must be isotopes. Is Sam correct? Explain your answer. A: No, isotopes have the same number of protons but different numbers of neutrons so they actually have different mass numbers.

8 Hydrogen has three isotopes, hydrogen-1, hydrogen-2 and hydrogen-3. Explain what an isotope is and the similarities and differences between these three isotopes. A: Isotopes contain the same number of protons but different numbers of neutrons – they have the same atomic number. All three isotopes of hydrogen contain one proton. Hydrogen-1 does not contain any neutrons. Hydrogen-2 contains one neutron. Hydrogen-3 contains two neutrons.