12 7 NOTES Equilibrium Chemical Equilibrium Reactions are

12. 7 – NOTES Equilibrium

Chemical Equilibrium • Reactions are Reversible! • Most reactions (with exceptions) are able to reverse back to the original reactants. • In reality, BOTH reactions (forward and backward) occur at the same time.


• Reaction Kinetics • In order for a reaction to occur successfully, it is dependent upon the number of successful collisions that occur. If molecules are not colliding with each other, then the reaction will not occur. • Reactions occur at different rates depending on a number of factors. • Concentration: As concentration increases, more molecules are present, the number of successful collisions increases, This will increase the rate of reaction. • Temperature: As temperature decreases, kinetic energy of the molecules decreases and the number of successful collisions decreases. This will decrease the rate of reaction. • Surface Area: As surface area increases (particle size increases) the ability for molecules to make contact increases. The number of successful collisions will increase and the rate of reaction increase. • Catalyst: a catalyst is a substance that when present, improves the ability of the reactants to collide. • As the concentration of a catalyst increases, successful collisions will increase, and the rate of reaction will increase.
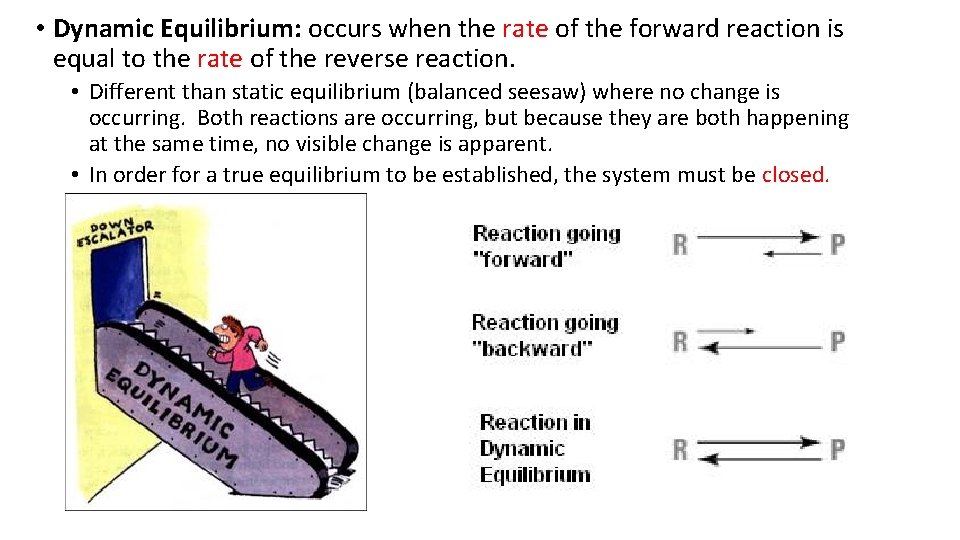
• Dynamic Equilibrium: occurs when the rate of the forward reaction is equal to the rate of the reverse reaction. • Different than static equilibrium (balanced seesaw) where no change is occurring. Both reactions are occurring, but because they are both happening at the same time, no visible change is apparent. • In order for a true equilibrium to be established, the system must be closed.
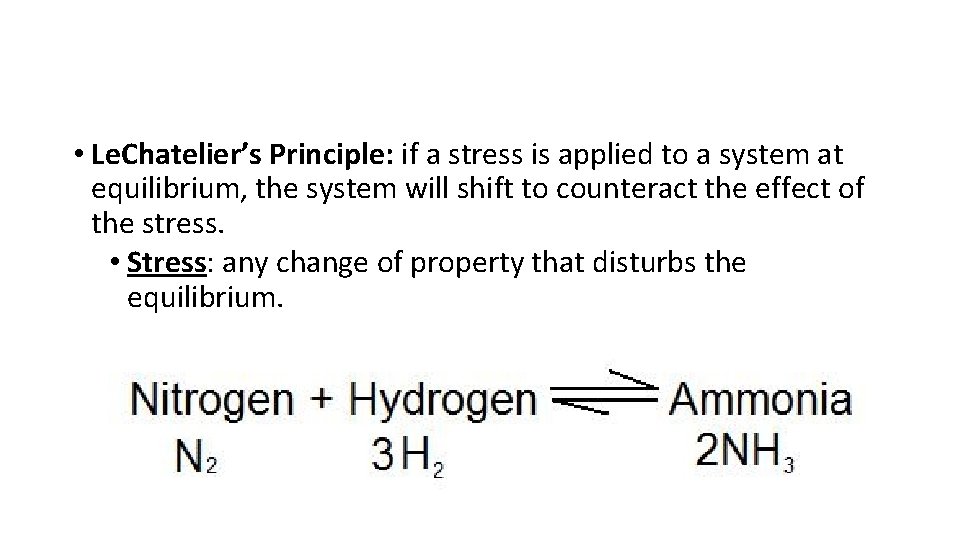
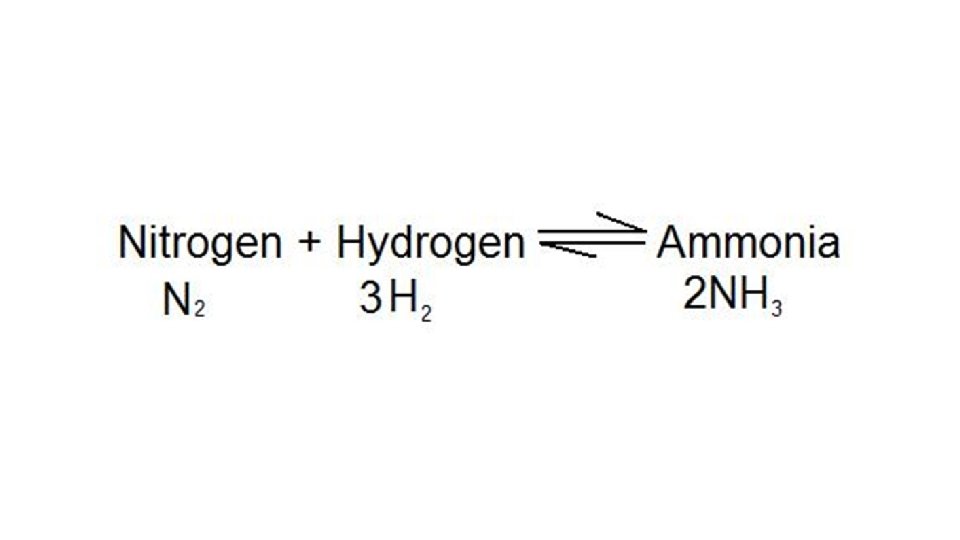
• Le. Chatelier’s Principle: if a stress is applied to a system at equilibrium, the system will shift to counteract the effect of the stress. • Stress: any change of property that disturbs the equilibrium.

• Effect of Concentration: the amount of a substance in a system • If any substance’s concentration is increased, the system must consume the extra amount at a faster rate. The system will shift away from the substance that was added. • If any substance’s concentration is decreased, the system must remove more of that substance. The system will shift toward the substance that was removed.
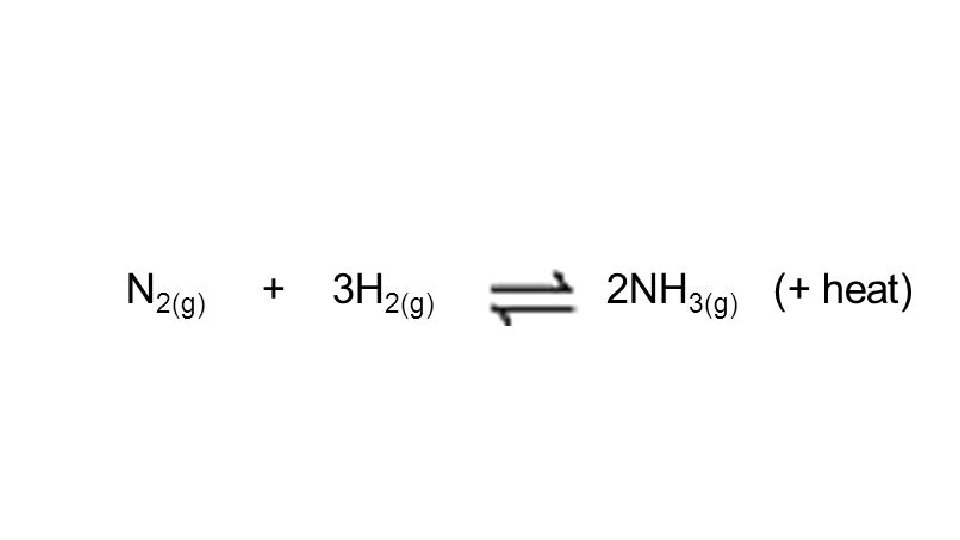
• Effect of Temperature • In order to analyze the effect of temperature, it must be known whether the reaction is endothermic or exothermic. Note: Unless otherwise stated, the statement “the reaction is endo/exothermic refers to the forward reaction! • If a reaction is endothermic, the “heat” term belongs on the left. • If a reaction is exothermic, the “heat” term belongs on the right. • If the temperature of a system is increased, the reaction must shift to consume the additional heat. The reaction will shift away from the “heat” term. • If the temperature of a system is decreased, the reaction must replace heat. The reaction will shift toward the “heat” term.

• Effect of a Catalyst • A catalyst’s job is to speed up a reaction. • When a catalyst is added to a system at equilibrium, it speeds up BOTH the forward and reverse reactions, so nothing occurs!

• General Rule: If a substance/heat is added, the system shifts away from whatever was added. If a substance/heat is removed, the system shifts toward whatever was removed.

• Examples: • Given the exothermic equilibrium: A + B C + D colorless orange green colorless • Predict the direction of shift and the color change given each of the following conditions: • Add extra of substance A: • • Place the system in an ice bath: • • Precipitate D out of the system: • • Place the system in hot water:
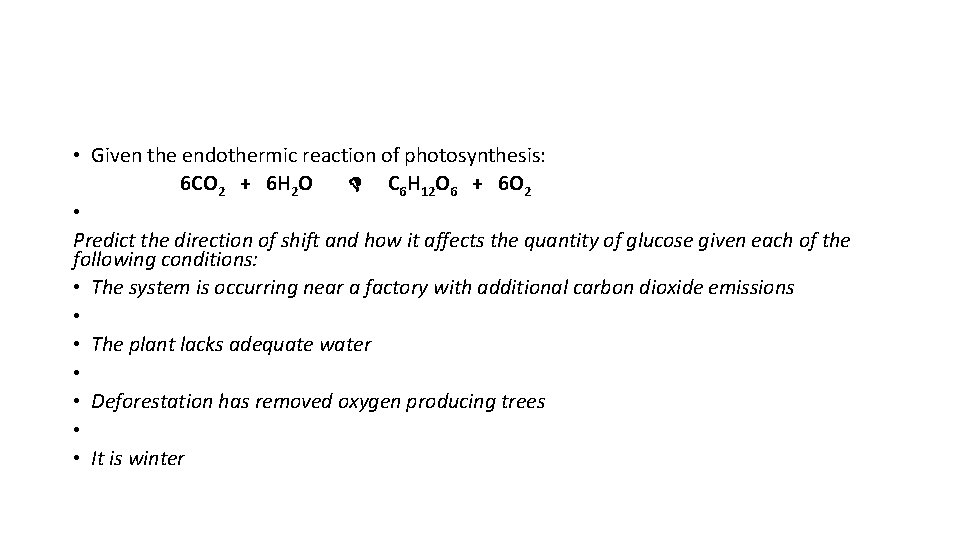
• Given the endothermic reaction of photosynthesis: 6 CO 2 + 6 H 2 O C 6 H 12 O 6 + 6 O 2 • Predict the direction of shift and how it affects the quantity of glucose given each of the following conditions: • The system is occurring near a factory with additional carbon dioxide emissions • • The plant lacks adequate water • • Deforestation has removed oxygen producing trees • • It is winter

- Slides: 15