12 6 NOTES Colligative Properties III Colligative Properties





- Slides: 14

12. 6 NOTES Colligative Properties

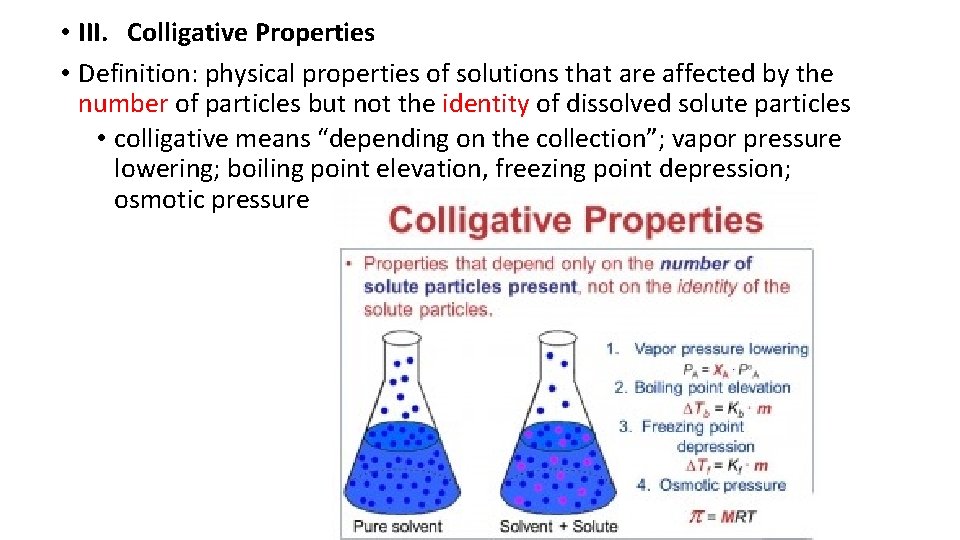
• III. Colligative Properties • Definition: physical properties of solutions that are affected by the number of particles but not the identity of dissolved solute particles • colligative means “depending on the collection”; vapor pressure lowering; boiling point elevation, freezing point depression; osmotic pressure

• A. Electrolytes and colligative properties • Ionic compounds are electrolytes, which means: • they dissociate in water to form a solution that conducts electricity • some molecular compounds will also ionize to conduct electricity (acids);

• Strong electrolytes: compounds that completely dissociate (do not produce an equilibrium); • Ex. dissolving 1 m of Na. Cl will produce 2 moles of ions while 1 m of Ca. Cl 2 will yield 3 moles of ions; • Weak electrolytes: compounds that only partially dissociate (equilibrium is established) • vinegar • Nonelectrolytes: a 1 m solution of sucrose would contain 1 mole of sucrose particles b/c sucrose does not ionize; • • Ca. Cl 2>Na. Cl>sucrose will have greater effect on colligative properties b/c has more particles!!

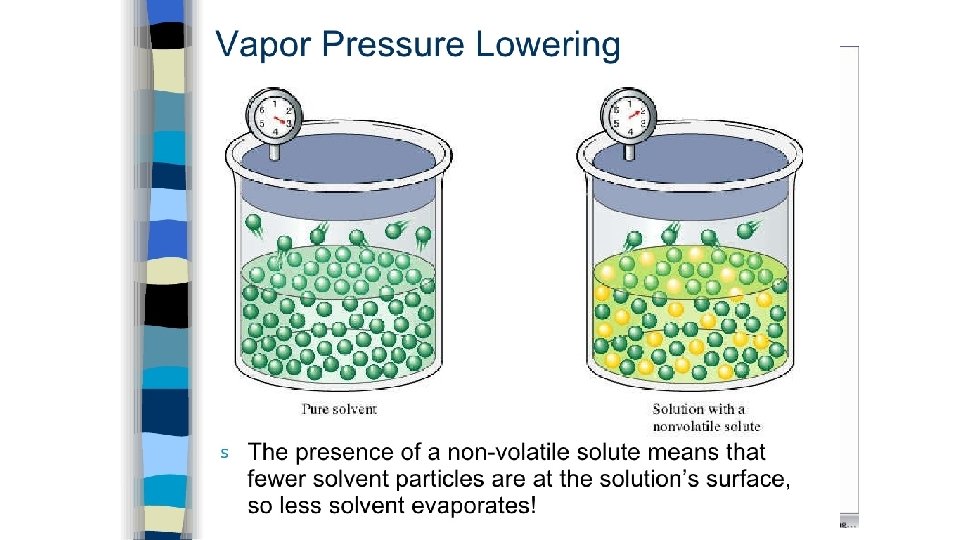
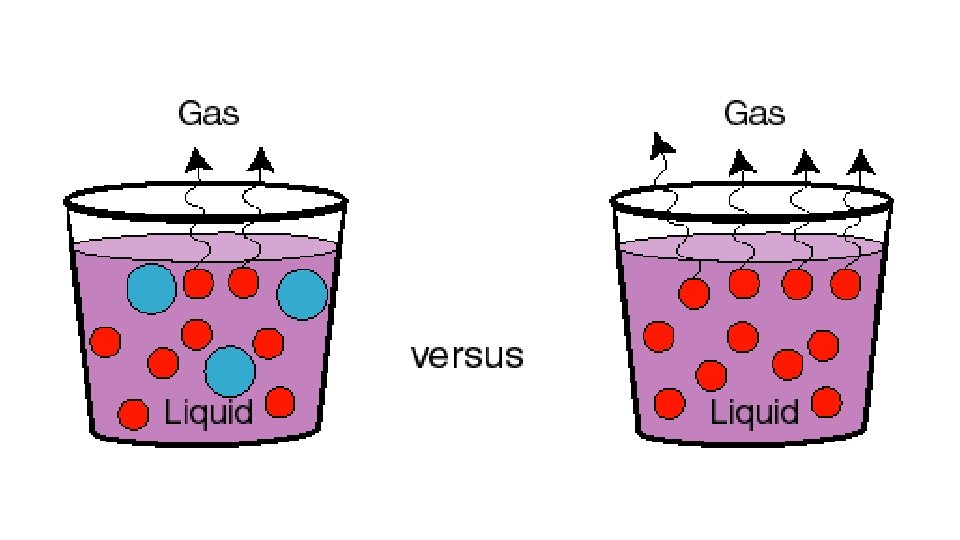
• Vapor pressure lowering - when a nonvolatile solute is added solute particles now appear at the surface of the liquid lowering the # of solvent particles at the surface • fewer solvent particles at the surface fewer solvent particles will enter the gaseous phase v. p. is lowered • greater # of solute particles, the lower the v. p. • electrolyte vs. nonelectrolyte = 1 mole of nonelectrolyte (sucrose & ethanol) will have the same effect, whereas Na. Cl, Na 2 SO 4, and Al. Cl 3 will have an increasingly greater effect b/c # of ions increases


• Boiling point elevation - recall that BP is dependent on vapor pressure • if vapor pressure is lowered, the solution will have to be heated to higher temperatures before the vapor pressure will equal the atmospheric pressure • Kb is a constant based on a 1 m nonvolatile, nonelectrolyte solution • Greater the number of solute particles, greater the BP elevation; • ∆Tb = Kbm for nonelectrolytes For water, Kb = 0. 52°/m • ∆Tb = Kbmi for electrolytes i = number of solute particles


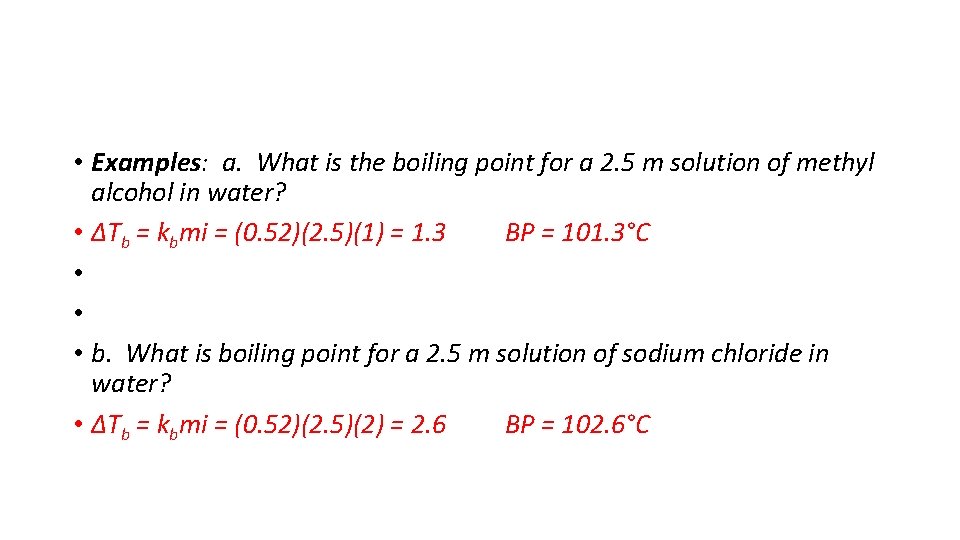
• Examples: a. What is the boiling point for a 2. 5 m solution of methyl alcohol in water? • ΔTb = kbmi = (0. 52)(2. 5)(1) = 1. 3 BP = 101. 3°C • • • b. What is boiling point for a 2. 5 m solution of sodium chloride in water? • ΔTb = kbmi = (0. 52)(2. 5)(2) = 2. 6 BP = 102. 6°C

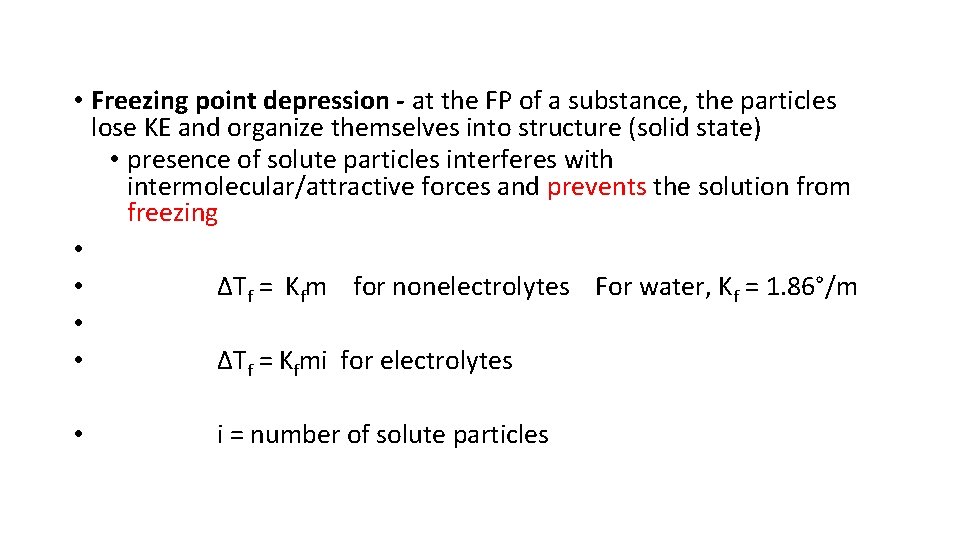
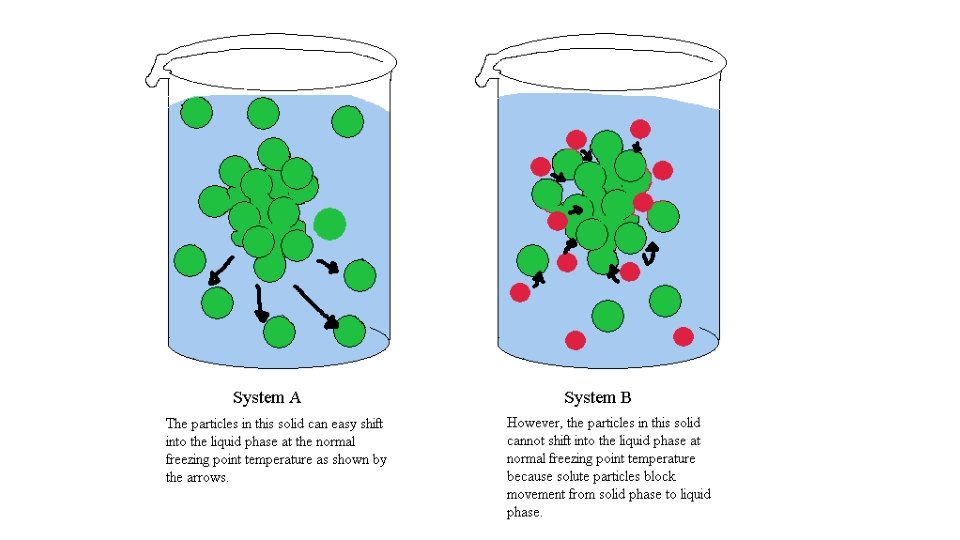
• Freezing point depression - at the FP of a substance, the particles lose KE and organize themselves into structure (solid state) • presence of solute particles interferes with intermolecular/attractive forces and prevents the solution from freezing • • ∆Tf = Kfm for nonelectrolytes For water, Kf = 1. 86°/m • • ∆Tf = Kfmi for electrolytes • i = number of solute particles


• Examples: a. What is the freezing point of a 3. 0 m solution of methyl alcohol in water? • ΔTf = kfmi = (1. 86)(3. 0)(1) = 5. 6 FP = -5. 6°C • • b. What is the freezing point of a 3. 0 m solution of calcium chloride in water? • ΔTf = kfmi = (1. 86)(3. 0)(3) = 17 FP = -17°C

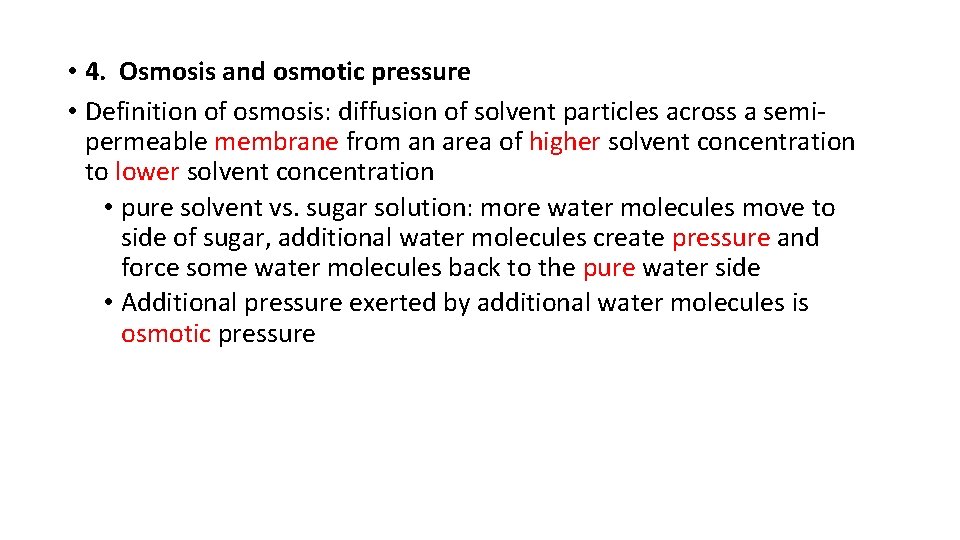
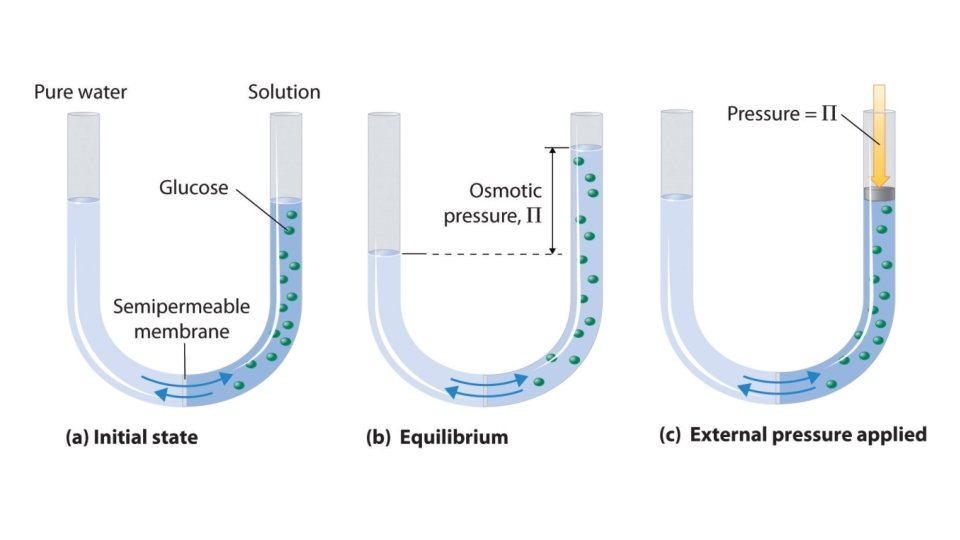
• 4. Osmosis and osmotic pressure • Definition of osmosis: diffusion of solvent particles across a semipermeable membrane from an area of higher solvent concentration to lower solvent concentration • pure solvent vs. sugar solution: more water molecules move to side of sugar, additional water molecules create pressure and force some water molecules back to the pure water side • Additional pressure exerted by additional water molecules is osmotic pressure
