12 3 Limiting Reagent and Percent Yield 12






- Slides: 13

12. 3 Limiting Reagent and Percent Yield

12. 3 Objectives Identify the limiting reagent in a reaction. Calculate theoretical yield, actual yield, or percent yield.

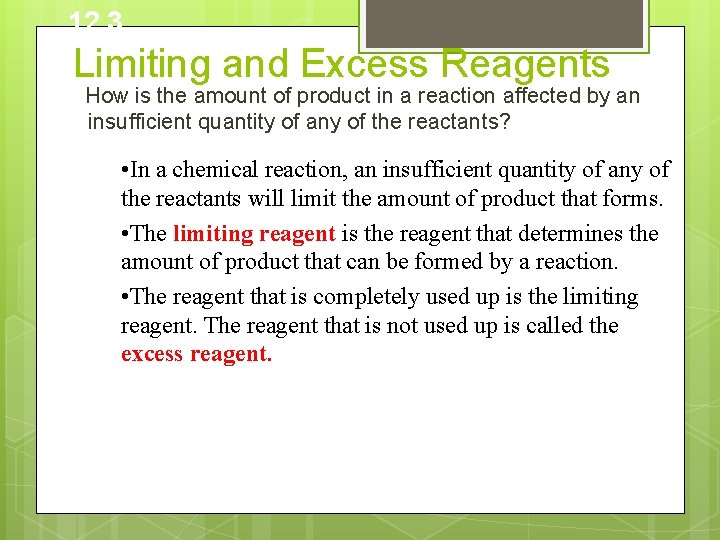
12. 3 Limiting and Excess Reagents How is the amount of product in a reaction affected by an insufficient quantity of any of the reactants? • In a chemical reaction, an insufficient quantity of any of the reactants will limit the amount of product that forms. • The limiting reagent is the reagent that determines the amount of product that can be formed by a reaction. • The reagent that is completely used up is the limiting reagent. The reagent that is not used up is called the excess reagent.



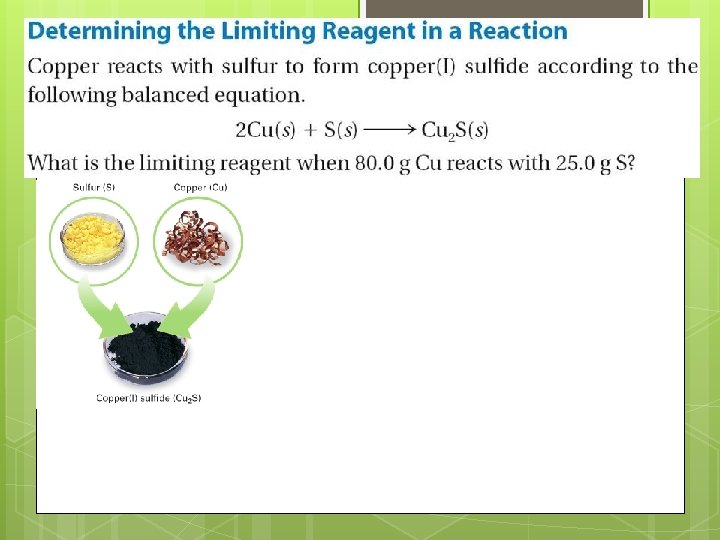
Finding Excess Reactant Using the Calculation from before we can see that Sulfur is the reactant that was in excess. However, sometimes we will still need to find out how much excess we have.

Excess continued… We can find the excess by: Converting the moles NEEDED into grams Subtracting the previous step from the amount of grams of substance given In the case of Sulfur: Given: 25. 0 g =. 779 moles Needed. 630 moles (to convert multiply by molar mass, 32. 066 g/mol), 20. 2 grams Subtract: 25 grams – 20. 2 grams = 4. 8 grams excess S

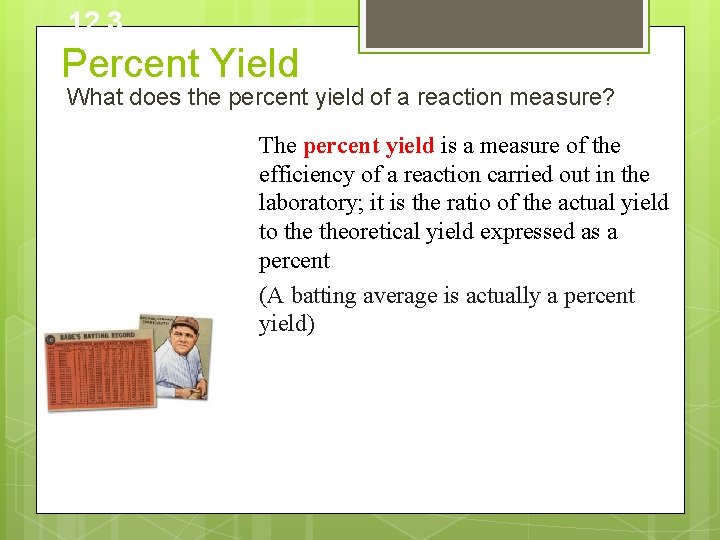
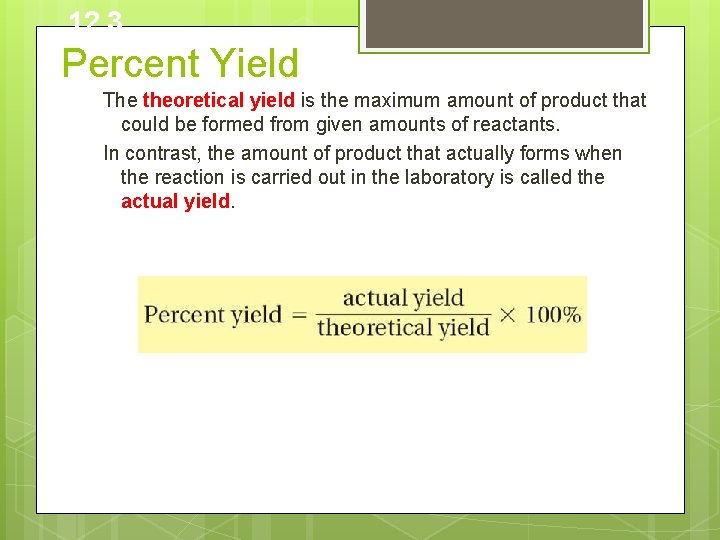
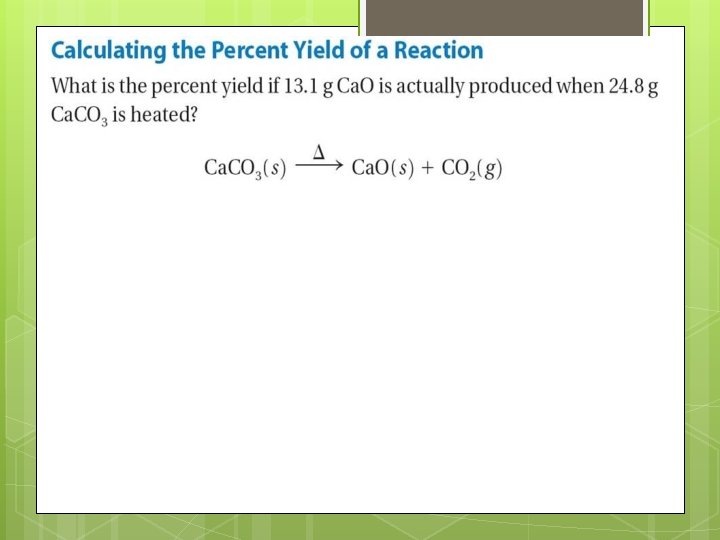
12. 3 Percent Yield What does the percent yield of a reaction measure? The percent yield is a measure of the efficiency of a reaction carried out in the laboratory; it is the ratio of the actual yield to theoretical yield expressed as a percent (A batting average is actually a percent yield)

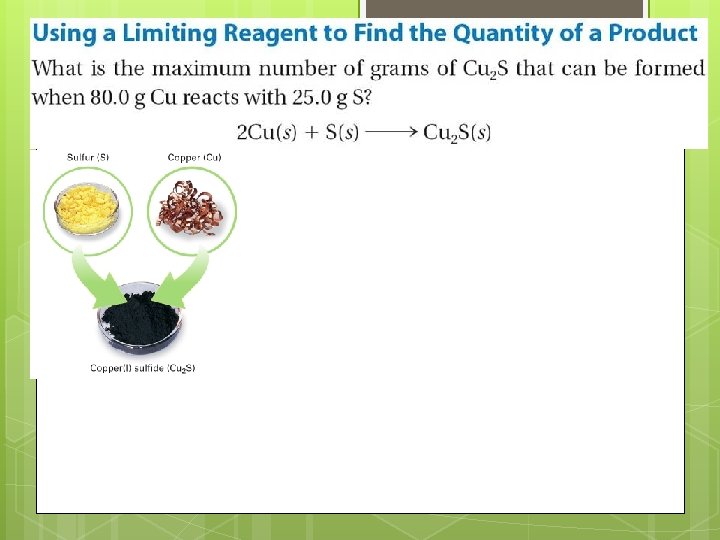
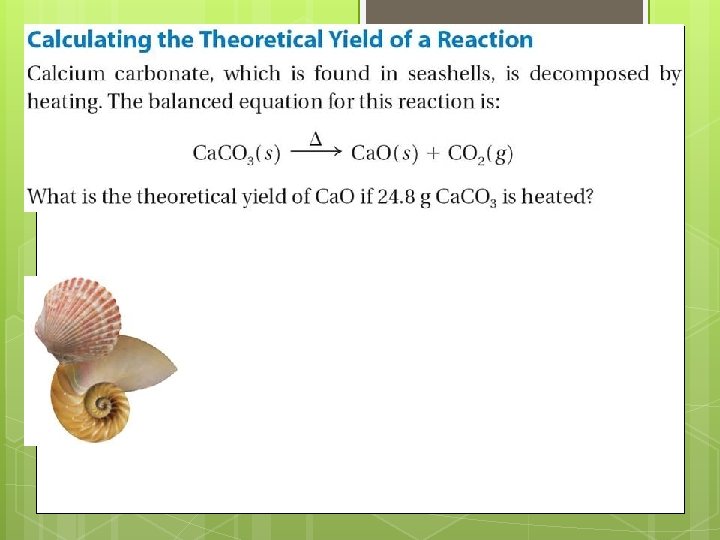
12. 3 Percent Yield The theoretical yield is the maximum amount of product that could be formed from given amounts of reactants. In contrast, the amount of product that actually forms when the reaction is carried out in the laboratory is called the actual yield.



Practice Problems Write all possible mole ratios for the following chemical equation. 2 Hg. O (s) 2 Hg (l) + O 2 (g)

Practice Problems Hydrogen gas can be produced through the following reaction. Mg (s) + 2 HCl (aq) Mg. Cl 2 (aq) + H 2 (g) What mass of HCl is consumed by the reaction of 2. 50 mol of magnesium?