12 1 NOTES Polarity and Intermolecular Bonding Review

12. 1 NOTES Polarity and Intermolecular Bonding Review
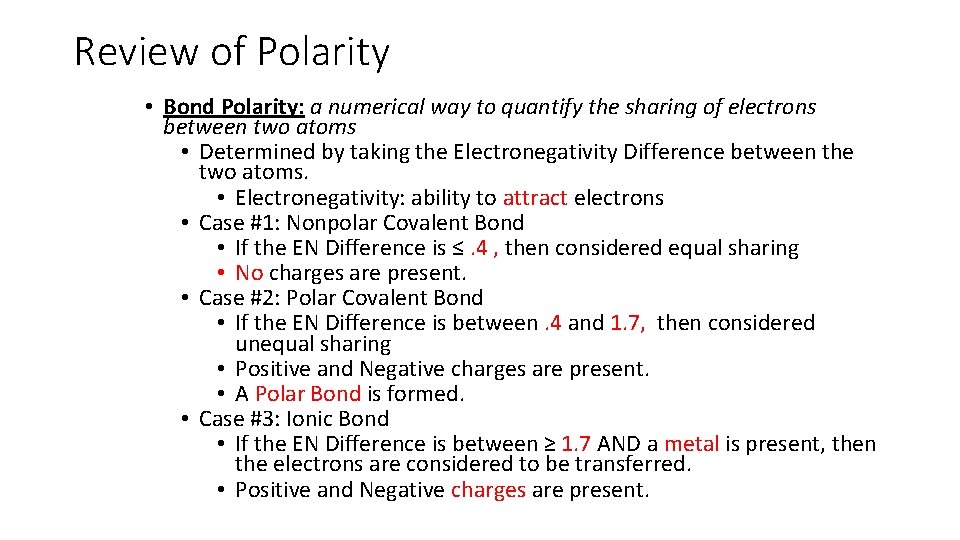
Review of Polarity • Bond Polarity: a numerical way to quantify the sharing of electrons between two atoms • Determined by taking the Electronegativity Difference between the two atoms. • Electronegativity: ability to attract electrons • Case #1: Nonpolar Covalent Bond • If the EN Difference is ≤. 4 , then considered equal sharing • No charges are present. • Case #2: Polar Covalent Bond • If the EN Difference is between. 4 and 1. 7, then considered unequal sharing • Positive and Negative charges are present. • A Polar Bond is formed. • Case #3: Ionic Bond • If the EN Difference is between ≥ 1. 7 AND a metal is present, then the electrons are considered to be transferred. • Positive and Negative charges are present.
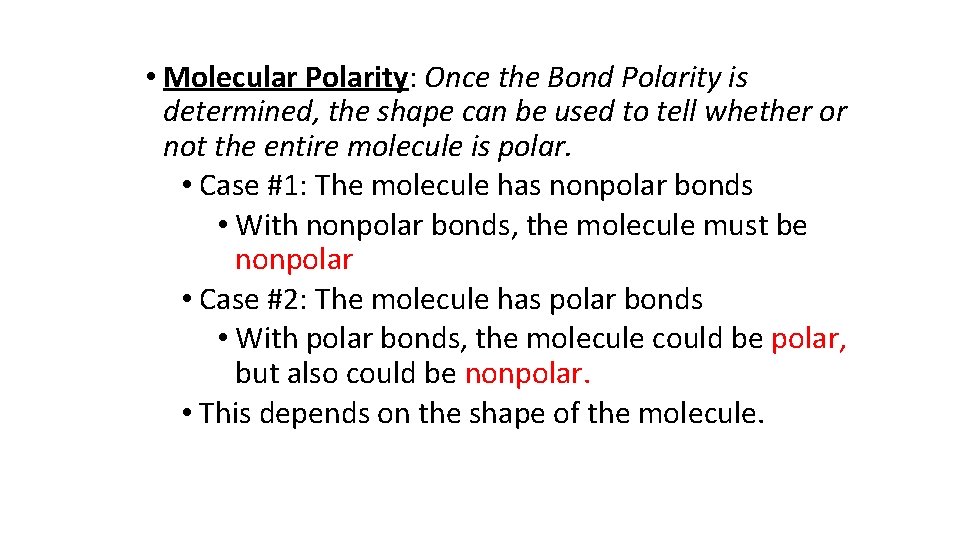
• Molecular Polarity: Once the Bond Polarity is determined, the shape can be used to tell whether or not the entire molecule is polar. • Case #1: The molecule has nonpolar bonds • With nonpolar bonds, the molecule must be nonpolar • Case #2: The molecule has polar bonds • With polar bonds, the molecule could be polar, but also could be nonpolar. • This depends on the shape of the molecule.
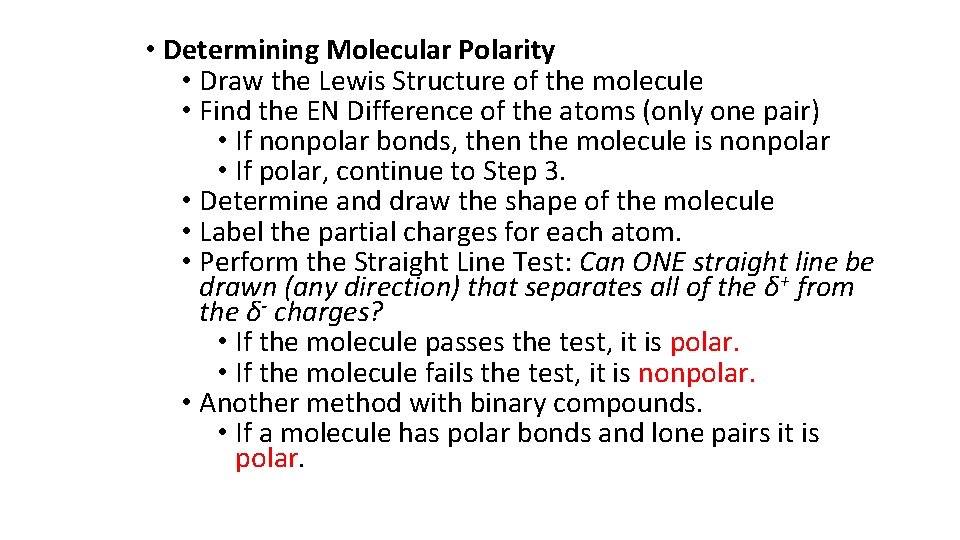
• Determining Molecular Polarity • Draw the Lewis Structure of the molecule • Find the EN Difference of the atoms (only one pair) • If nonpolar bonds, then the molecule is nonpolar • If polar, continue to Step 3. • Determine and draw the shape of the molecule • Label the partial charges for each atom. • Perform the Straight Line Test: Can ONE straight line be drawn (any direction) that separates all of the δ+ from the δ- charges? • If the molecule passes the test, it is polar. • If the molecule fails the test, it is nonpolar. • Another method with binary compounds. • If a molecule has polar bonds and lone pairs it is polar.
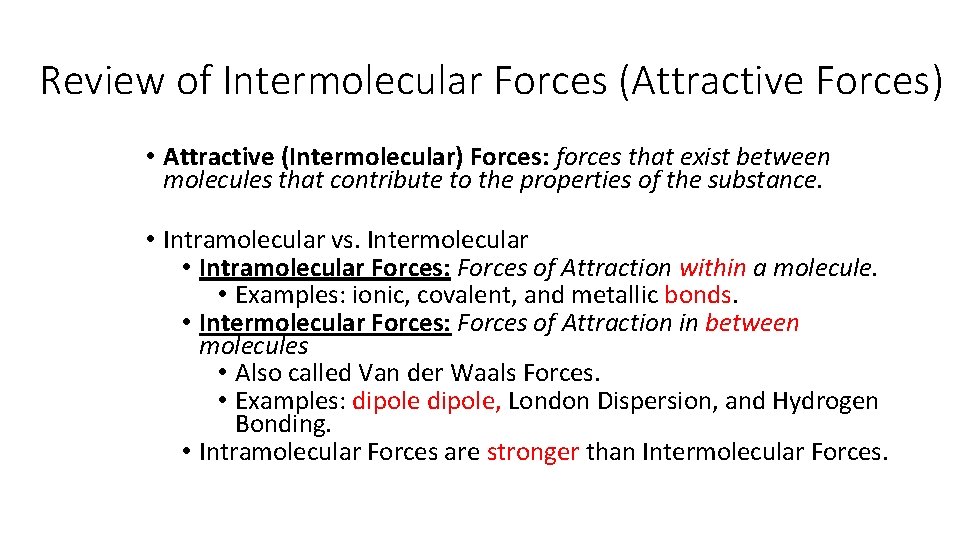
Review of Intermolecular Forces (Attractive Forces) • Attractive (Intermolecular) Forces: forces that exist between molecules that contribute to the properties of the substance. • Intramolecular vs. Intermolecular • Intramolecular Forces: Forces of Attraction within a molecule. • Examples: ionic, covalent, and metallic bonds. • Intermolecular Forces: Forces of Attraction in between molecules • Also called Van der Waals Forces. • Examples: dipole, London Dispersion, and Hydrogen Bonding. • Intramolecular Forces are stronger than Intermolecular Forces.
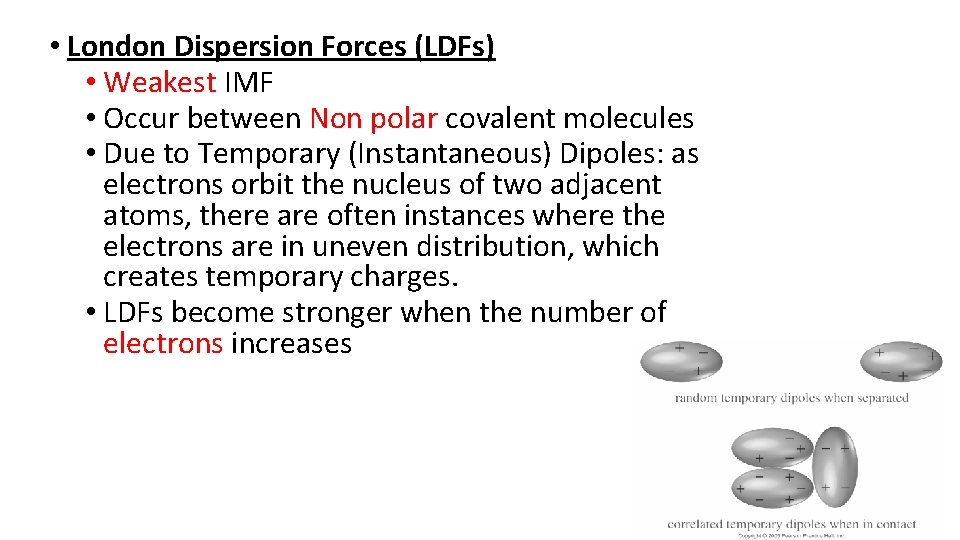
• London Dispersion Forces (LDFs) • Weakest IMF • Occur between Non polar covalent molecules • Due to Temporary (Instantaneous) Dipoles: as electrons orbit the nucleus of two adjacent atoms, there are often instances where the electrons are in uneven distribution, which creates temporary charges. • LDFs become stronger when the number of electrons increases

• Dipole-Dipole Forces • Stronger than LDFs • Occur due to Permanent Dipoles which are found in all polar covalent molecules. • How is this determined? Electronegativity Difference and Shape • The higher the EN Difference, the stronger the dipole-dipole interaction.

• Hydrogen Bonding • Strongest IMF • A special type of dipole-dipole force • Occur in Polar covalent molecules when the EN difference is very large and the atoms are very small. • Hydrogen must be bonded to either fluorine, oxygen, or nitrogen. • Chlorine is too big!
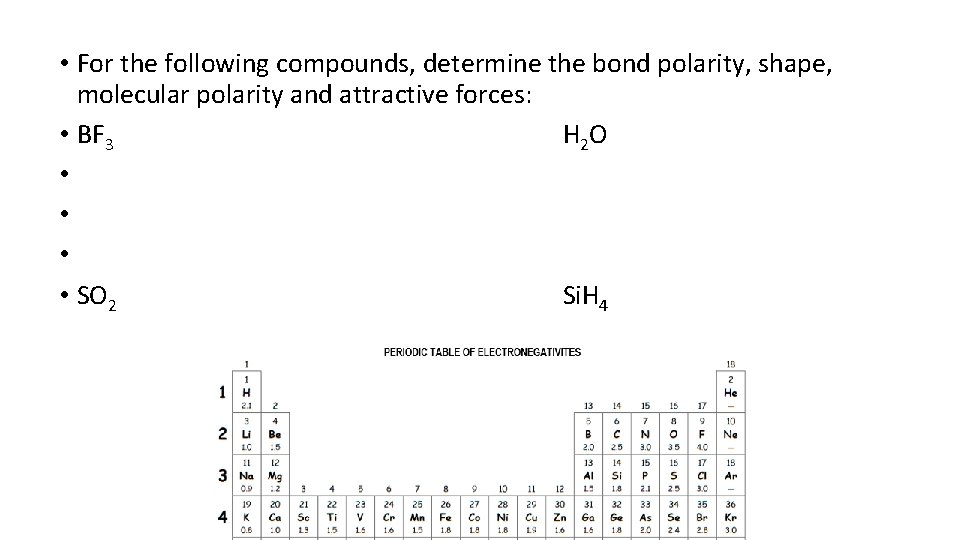
• For the following compounds, determine the bond polarity, shape, molecular polarity and attractive forces: • BF 3 H 2 O • • SO 2 Si. H 4
- Slides: 9