12 1 Atomic Structure Subatomic Particles Subatomic particles

12. 1 Atomic Structure
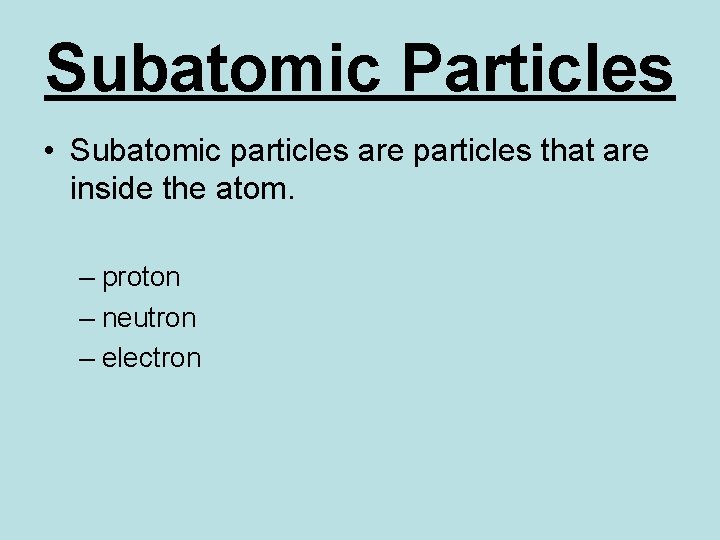
Subatomic Particles • Subatomic particles are particles that are inside the atom. – proton – neutron – electron
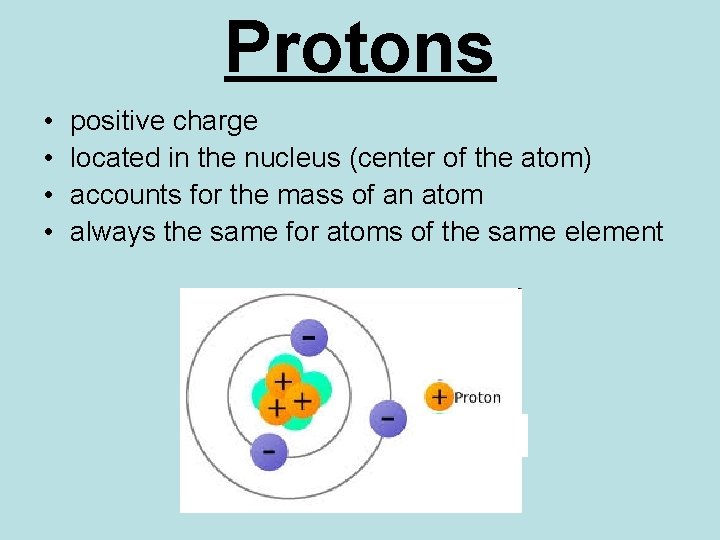
Protons • • positive charge located in the nucleus (center of the atom) accounts for the mass of an atom always the same for atoms of the same element
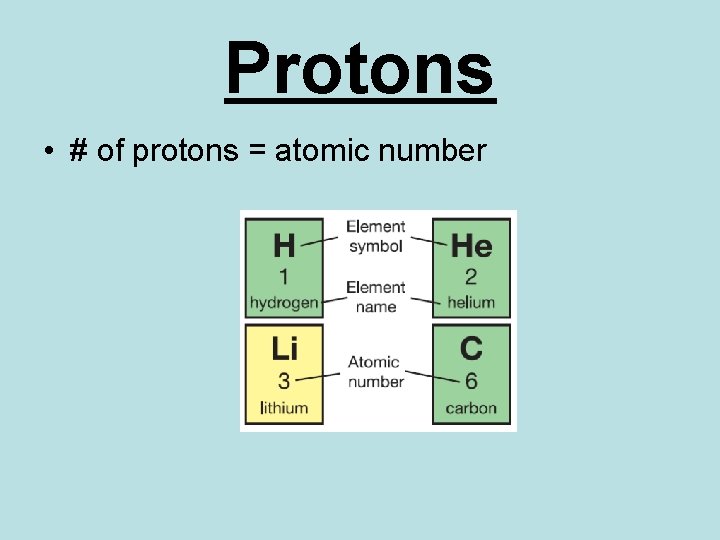
Protons • # of protons = atomic number
Check in: • How many protons are in the atoms of the following elements? –O – He –C – Al • Do the number of protons in an atom ever change?
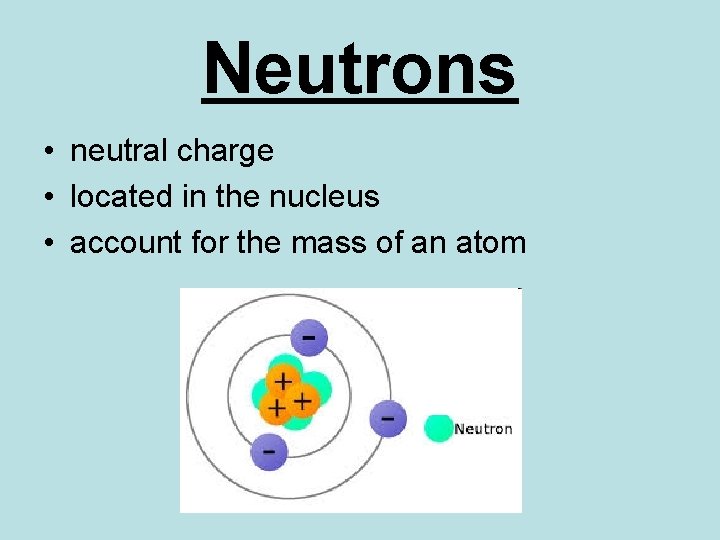
Neutrons • neutral charge • located in the nucleus • account for the mass of an atom
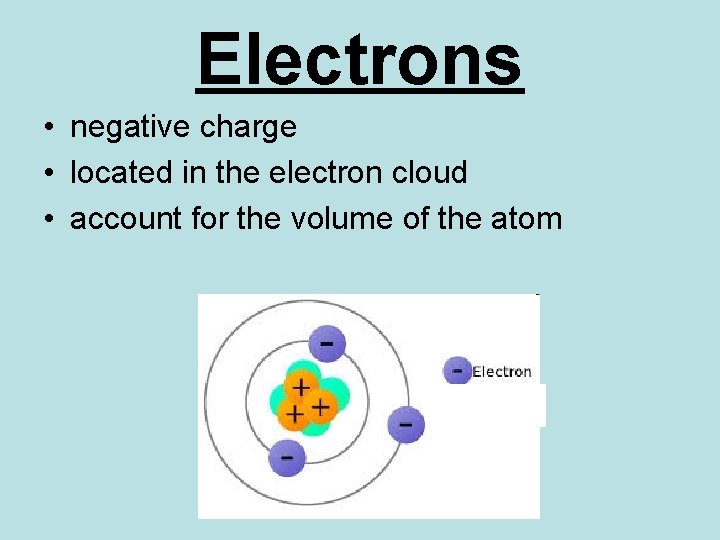
Electrons • negative charge • located in the electron cloud • account for the volume of the atom
Comparing Particles • protons and neutrons are MASSIVE compared to electrons – electrons mass is so small we tend to ignore it • to determine the mass of an atom (mass number) mass # = protons + neutrons

Check-in: • If an atom of oxygen has 8 protons and 7 neutrons, what is its mass? • If an atom of chlorine has a mass number of 36, how many neutrons does it have?

Atomic Forces REVIEW: • opposites attract • likes repel Protons and Electrons: • opposite charges – attract • attraction holds electrons onto the outside of the atom

Atomic Forces • Think: If like repel, then why are the protons together in the nucleus? • The strong force holds protons together in the nucleus.

VIDEO: Just How Small is the Atom? ? ? • http: //ed. ted. com/lessons/just-how-small-is -an-atom#watch
Varying Subatomic Particles • PROTONS NEVER CHANGE FOR ATOMS OF THE SAME ELEMENT! • electrons and neutrons, however, CAN change
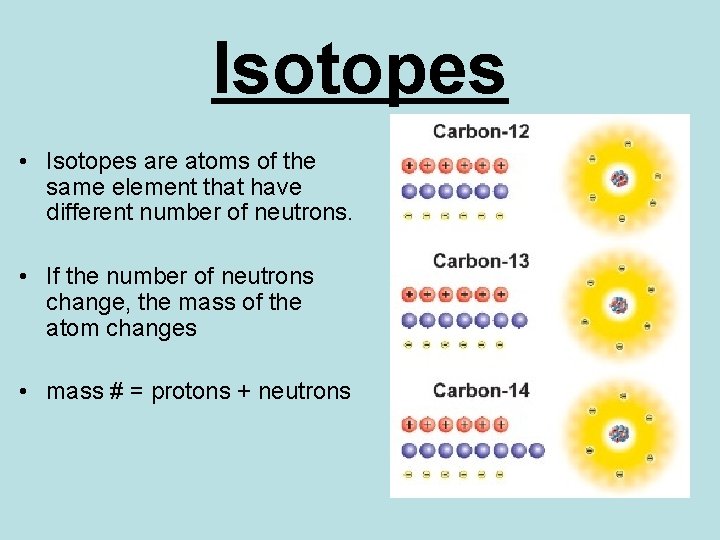
Isotopes • Isotopes are atoms of the same element that have different number of neutrons. • If the number of neutrons change, the mass of the atom changes • mass # = protons + neutrons
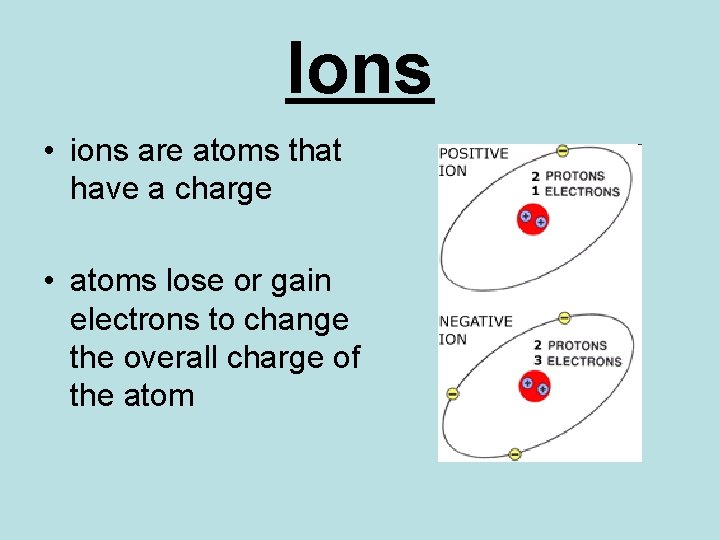
Ions • ions are atoms that have a charge • atoms lose or gain electrons to change the overall charge of the atom
- Slides: 15