11 Reactions in Aqueous Solutions II Calculations 1











- Slides: 36

11 Reactions in Aqueous Solutions II: Calculations 1

Chapter Goals 1. 2. 3. 4. 5. 6. Aqueous Acid-Base Reactions Calculations Involving Molarity Titrations Calculations for Acid-Base Titrations Oxidation-Reduction Reactions Balancing Redox Equations Adding in H+, OH- , or H 2 O to Balance Oxygen or Hydrogen Calculations for Redox Titrations 2

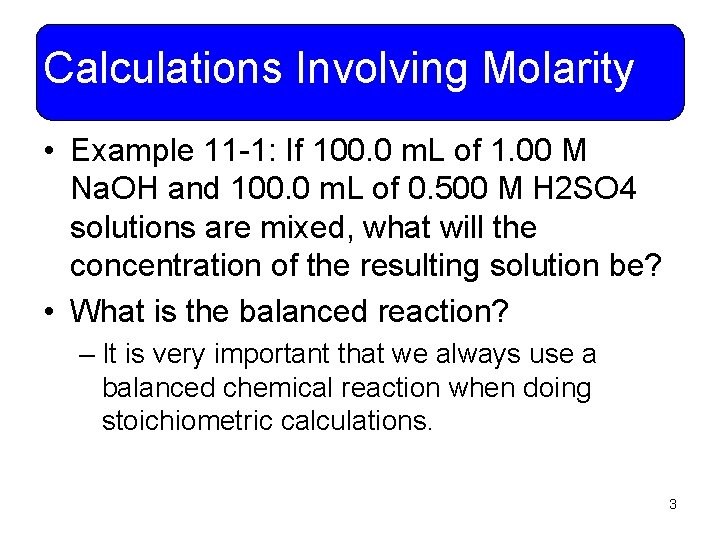
Calculations Involving Molarity • Example 11 -1: If 100. 0 m. L of 1. 00 M Na. OH and 100. 0 m. L of 0. 500 M H 2 SO 4 solutions are mixed, what will the concentration of the resulting solution be? • What is the balanced reaction? – It is very important that we always use a balanced chemical reaction when doing stoichiometric calculations. 3

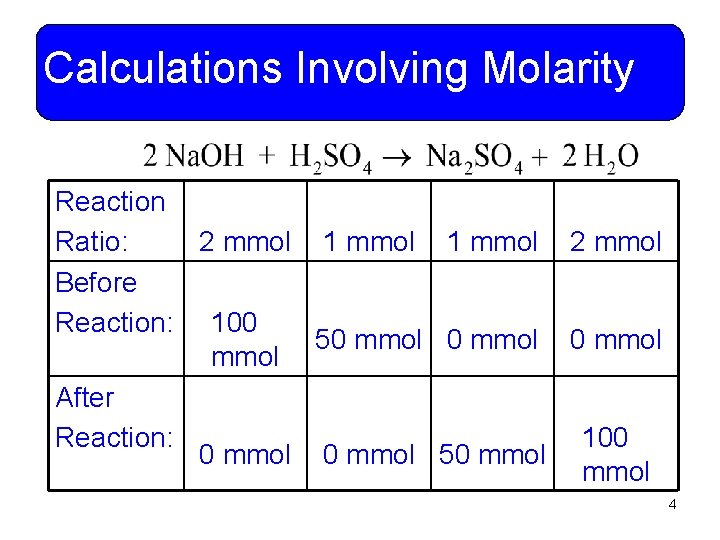
Calculations Involving Molarity Reaction Ratio: 2 mmol 1 mmol 2 mmol Before Reaction: 100 50 mmol After Reaction: 100 0 mmol 50 mmol 4

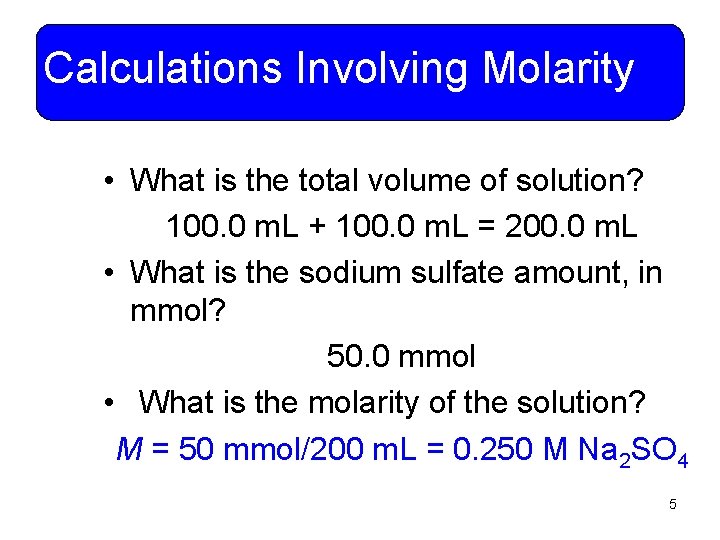
Calculations Involving Molarity • What is the total volume of solution? 100. 0 m. L + 100. 0 m. L = 200. 0 m. L • What is the sodium sulfate amount, in mmol? 50. 0 mmol • What is the molarity of the solution? M = 50 mmol/200 m. L = 0. 250 M Na 2 SO 4 5

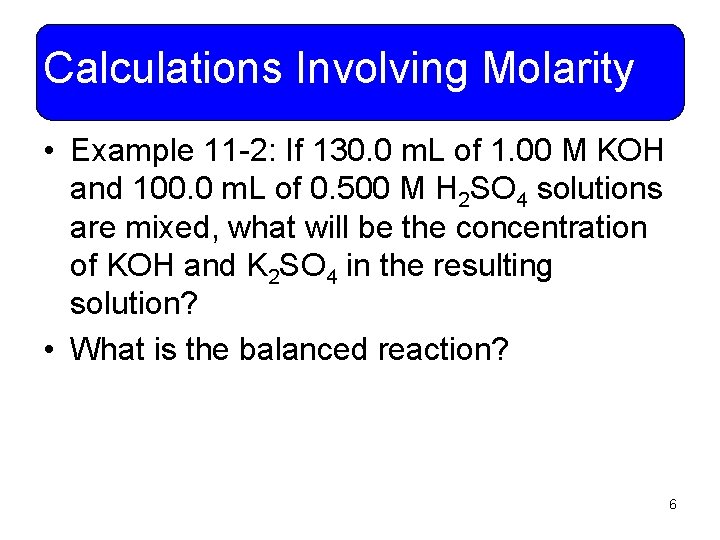
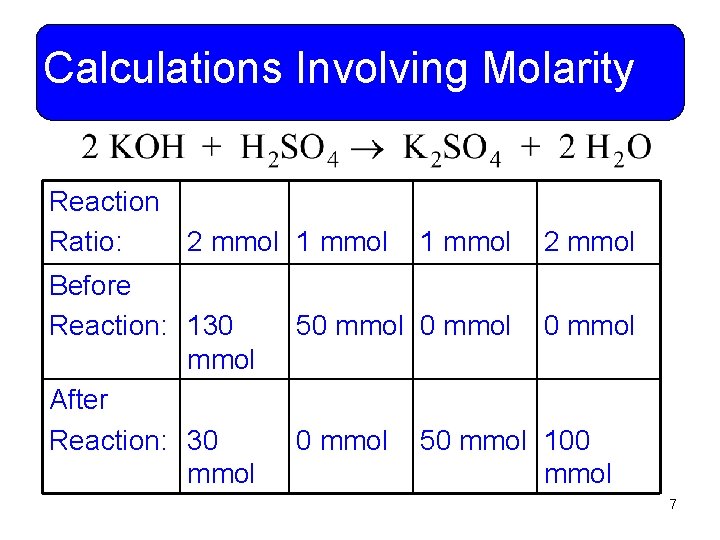
Calculations Involving Molarity • Example 11 -2: If 130. 0 m. L of 1. 00 M KOH and 100. 0 m. L of 0. 500 M H 2 SO 4 solutions are mixed, what will be the concentration of KOH and K 2 SO 4 in the resulting solution? • What is the balanced reaction? 6

Calculations Involving Molarity Reaction Ratio: 2 mmol 1 mmol Before Reaction: 130 mmol After Reaction: 30 mmol 1 mmol 2 mmol 50 mmol 50 mmol 100 mmol 7

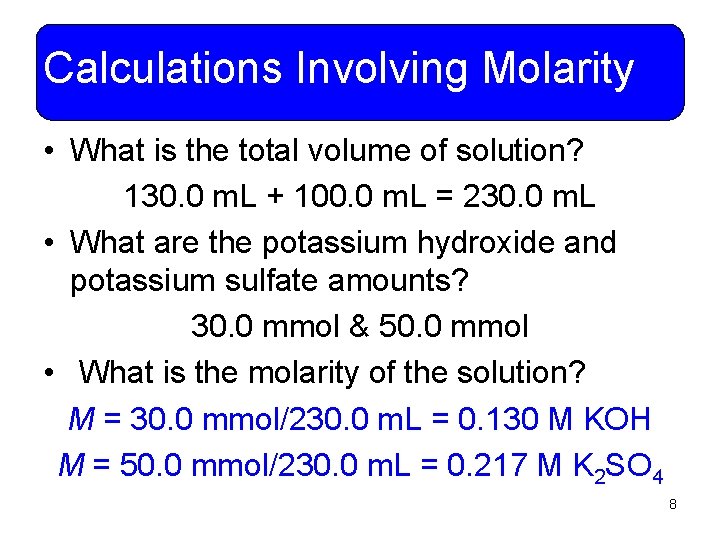
Calculations Involving Molarity • What is the total volume of solution? 130. 0 m. L + 100. 0 m. L = 230. 0 m. L • What are the potassium hydroxide and potassium sulfate amounts? 30. 0 mmol & 50. 0 mmol • What is the molarity of the solution? M = 30. 0 mmol/230. 0 m. L = 0. 130 M KOH M = 50. 0 mmol/230. 0 m. L = 0. 217 M K 2 SO 4 8

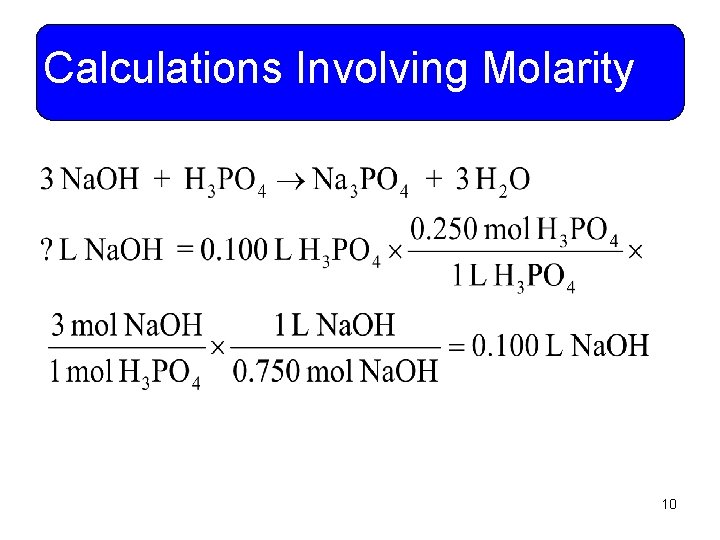
Calculations Involving Molarity • Example 11 -3: What volume of 0. 750 M Na. OH solution would be required to completely neutralize 100 m. L of 0. 250 M H 3 PO 4? You do it! 9

Calculations Involving Molarity 10

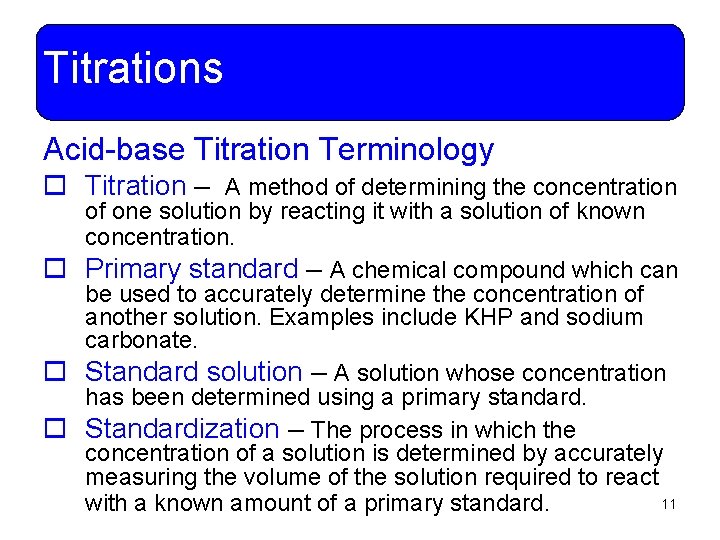
Titrations Acid-base Titration Terminology o Titration – A method of determining the concentration of one solution by reacting it with a solution of known concentration. o Primary standard – A chemical compound which can be used to accurately determine the concentration of another solution. Examples include KHP and sodium carbonate. o Standard solution – A solution whose concentration has been determined using a primary standard. o Standardization – The process in which the concentration of a solution is determined by accurately measuring the volume of the solution required to react 11 with a known amount of a primary standard.

Titrations Acid-base Titration Terminology o Indicator – A substance that exists in different forms with different colors depending on the concentration of the H+ in solution. Examples are phenolphthalein and bromothymol blue. o Equivalence point – The point at which stoichiometrically equivalent amounts of the acid and base have reacted. o End point – The point at which the indicator changes color and the titration is stopped. 12

Titrations Acid-base Titration Terminology 13

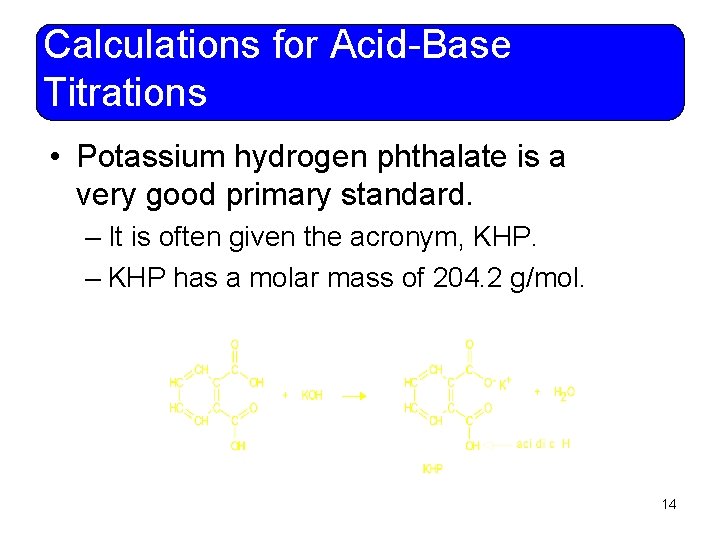
Calculations for Acid-Base Titrations • Potassium hydrogen phthalate is a very good primary standard. – It is often given the acronym, KHP. – KHP has a molar mass of 204. 2 g/mol. 14

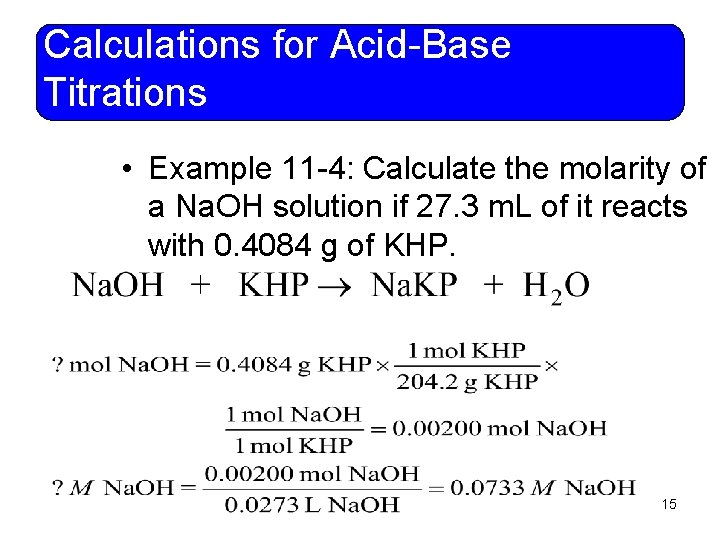
Calculations for Acid-Base Titrations • Example 11 -4: Calculate the molarity of a Na. OH solution if 27. 3 m. L of it reacts with 0. 4084 g of KHP. 15

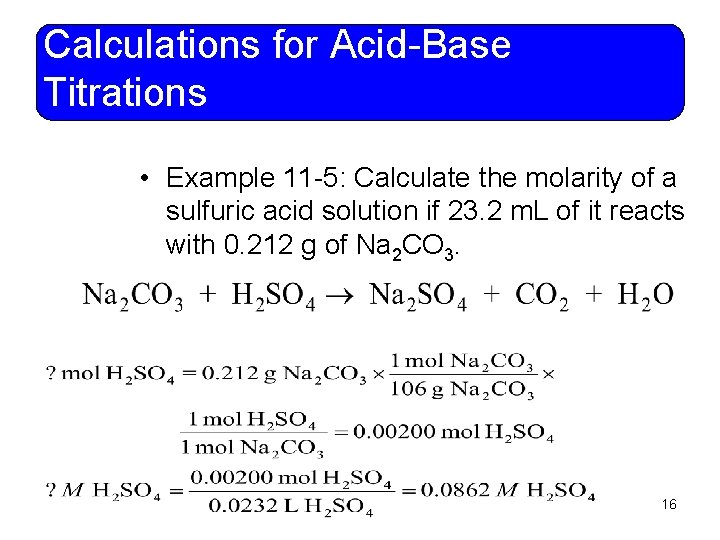
Calculations for Acid-Base Titrations • Example 11 -5: Calculate the molarity of a sulfuric acid solution if 23. 2 m. L of it reacts with 0. 212 g of Na 2 CO 3. 16

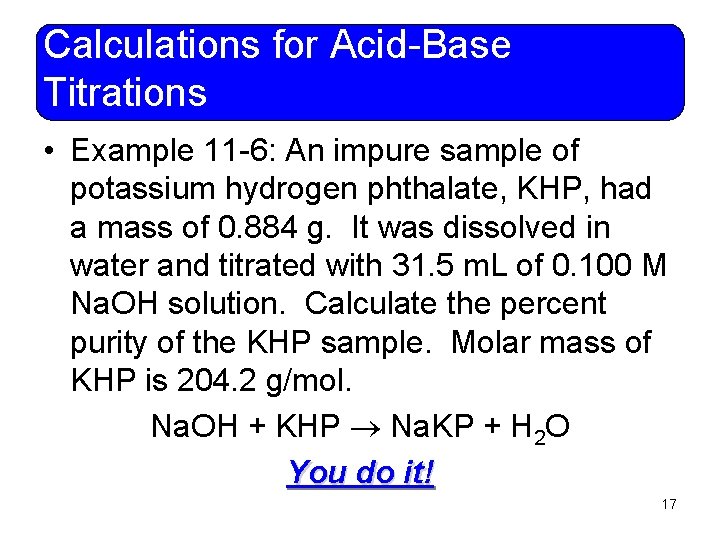
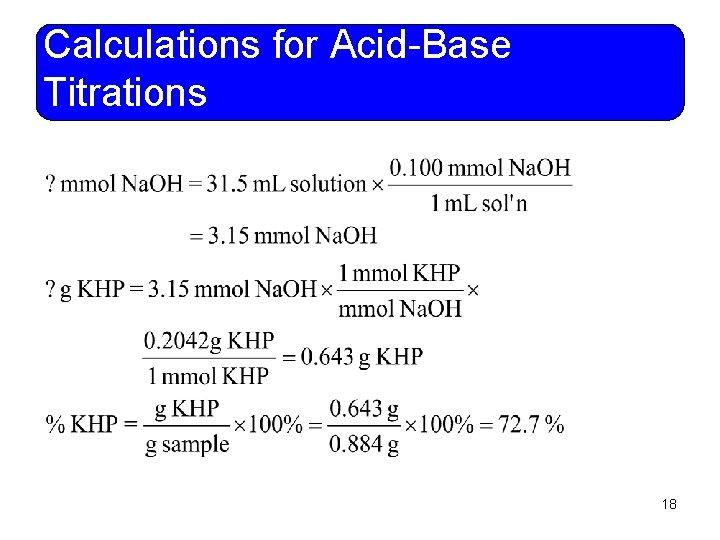
Calculations for Acid-Base Titrations • Example 11 -6: An impure sample of potassium hydrogen phthalate, KHP, had a mass of 0. 884 g. It was dissolved in water and titrated with 31. 5 m. L of 0. 100 M Na. OH solution. Calculate the percent purity of the KHP sample. Molar mass of KHP is 204. 2 g/mol. Na. OH + KHP Na. KP + H 2 O You do it! 17

Calculations for Acid-Base Titrations 18

Oxidation-Reduction Reactions • We have previously gone over the basic concepts of oxidation & reduction in Chapter 4. • Rules for assigning oxidation numbers were also introduced in Chapter 4. – Refresh your memory as necessary. • We shall learn to balance redox reactions using the half-reaction method. 19

Balancing Redox Reactions Half reaction method rules: 1. Write the unbalanced reaction. 2. Break the reaction into 2 half reactions: One oxidation half-reaction and One reduction half-reaction Each reaction must have complete formulas for molecules and ions. 3. Mass balance each half reaction by adding appropriate stoichiometric coefficients. To balance H and O we can add: § H+ or H 2 O in acidic solutions. § OH- or H 2 O in basic solutions. 20

Balancing Redox Reactions 4. Charge balance the half reactions by adding appropriate numbers of electrons. § Electrons will be products in the oxidation halfreaction. § Electrons will be reactants in the reduction halfreaction. 5. Multiply each half reaction by a number to make the number of electrons in the oxidation halfreaction equal to the number of electrons reduction half-reaction. 6. Add the two half reactions. 7. Eliminate any common terms and reduce coefficients to smallest whole numbers. 21

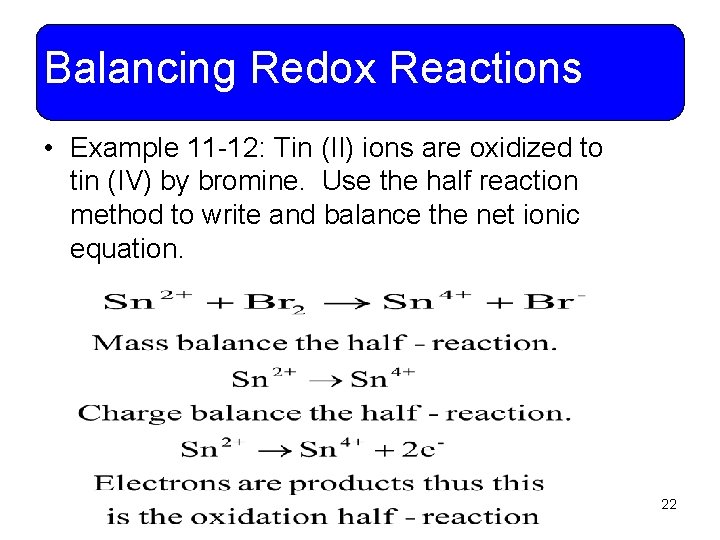
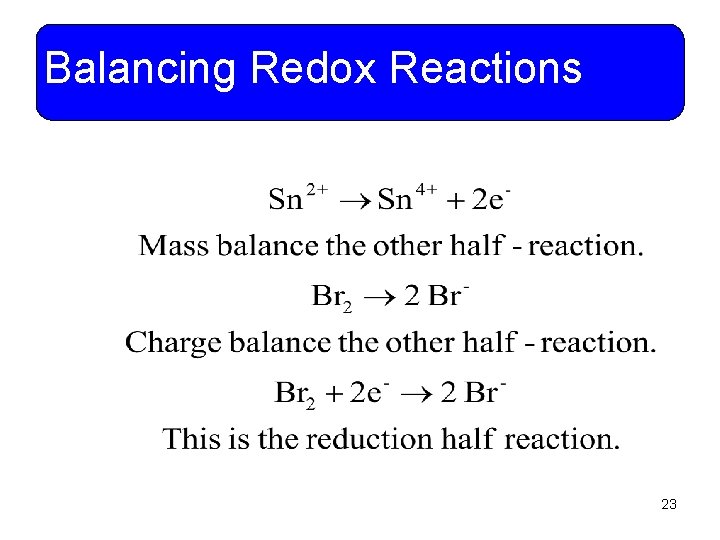
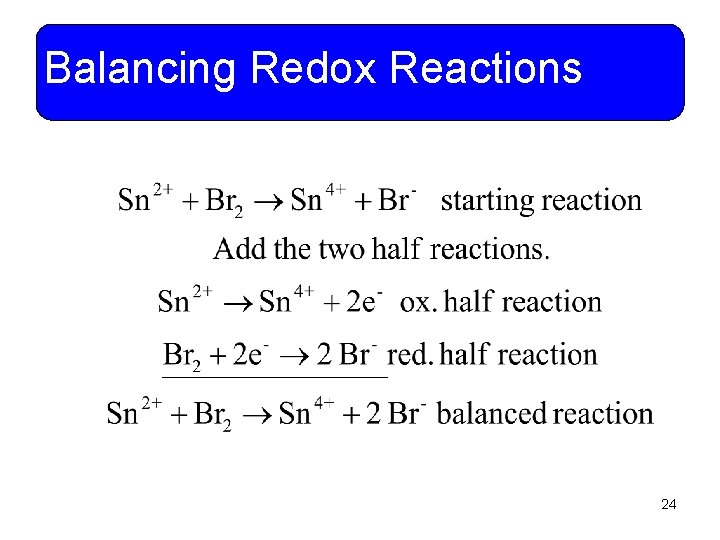
Balancing Redox Reactions • Example 11 -12: Tin (II) ions are oxidized to tin (IV) by bromine. Use the half reaction method to write and balance the net ionic equation. 22

Balancing Redox Reactions 23

Balancing Redox Reactions 24

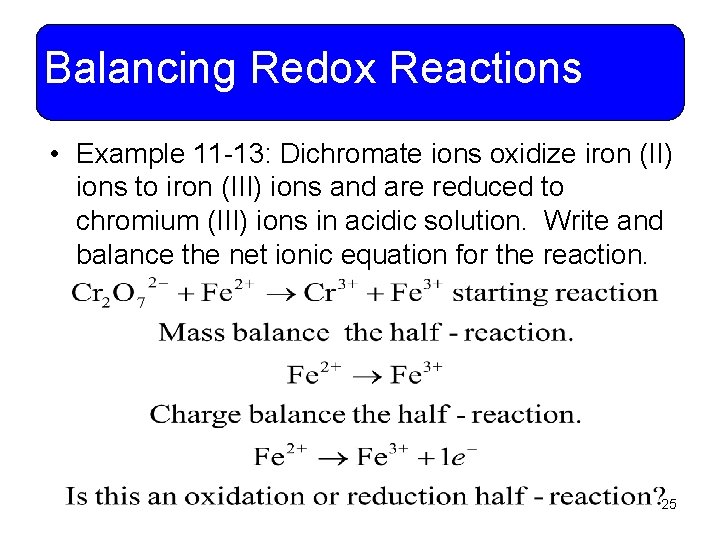
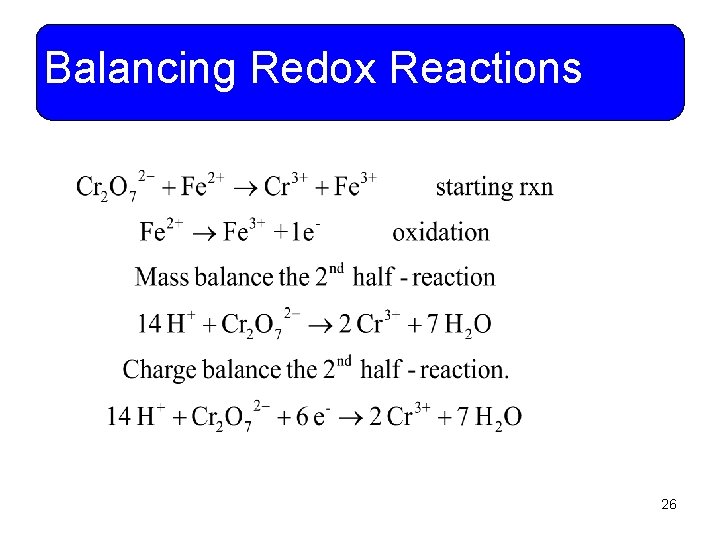
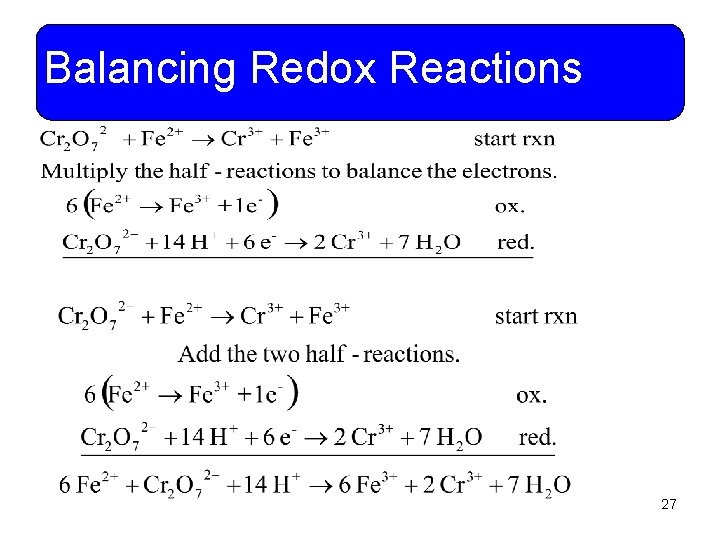
Balancing Redox Reactions • Example 11 -13: Dichromate ions oxidize iron (II) ions to iron (III) ions and are reduced to chromium (III) ions in acidic solution. Write and balance the net ionic equation for the reaction. 25

Balancing Redox Reactions 26

Balancing Redox Reactions 27

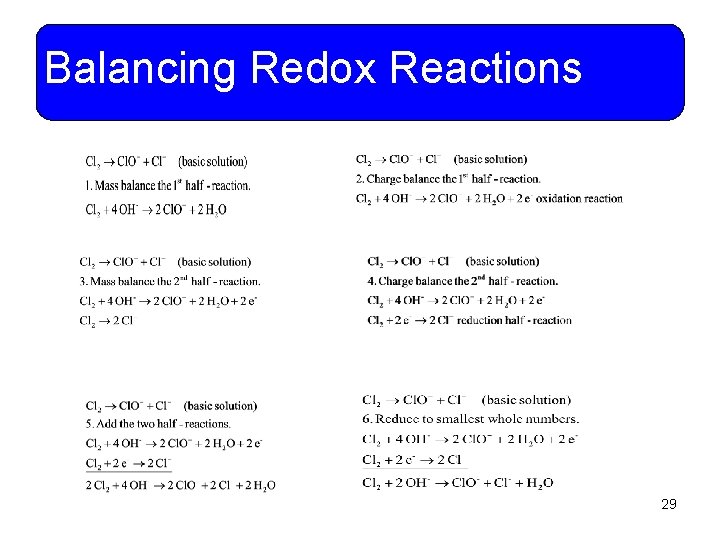
Balancing Redox Reactions • Example 11 -15: When chlorine is bubbled into basic solution, it forms hypochlorite ions and chloride ions. Write and balance the net ionic equation. You do it! • This is a disproportionation redox reaction. The same species, in this case Cl 2, is both reduced and oxidized. 28

Balancing Redox Reactions 29

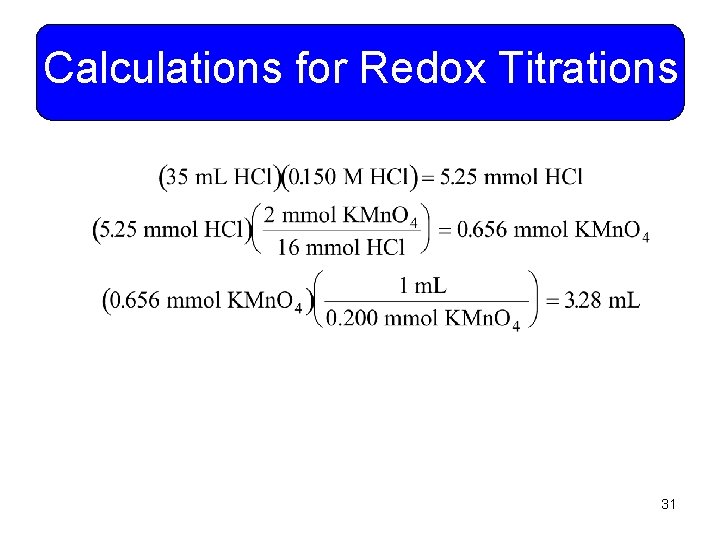
Calculations for Redox Titrations • Just as we have done stoichiometry with acid-base reactions, it can also be done with redox reactions. • Example 11 -16: What volume of 0. 200 M KMn. O 4 is required to oxidize 35. 0 m. L of 0. 150 M HCl? The balanced reaction is: You do it! 30

Calculations for Redox Titrations 31

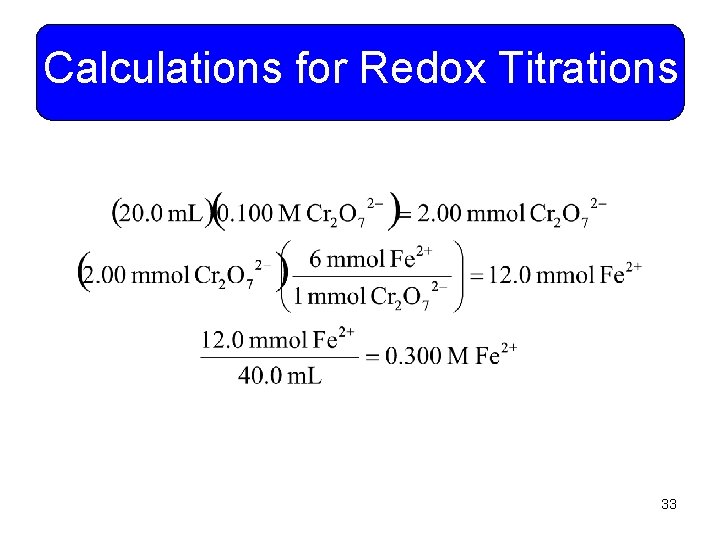
Calculations for Redox Titrations • Example 11 -17: A volume of 40. 0 m. L of iron (II) sulfate is oxidized to iron (III) by 20. 0 m. L of 0. 100 M potassium dichromate solution. What is the concentration of the iron (II) sulfate solution? From Example 11 -19 the balanced equation is: You do it! 32

Calculations for Redox Titrations 33

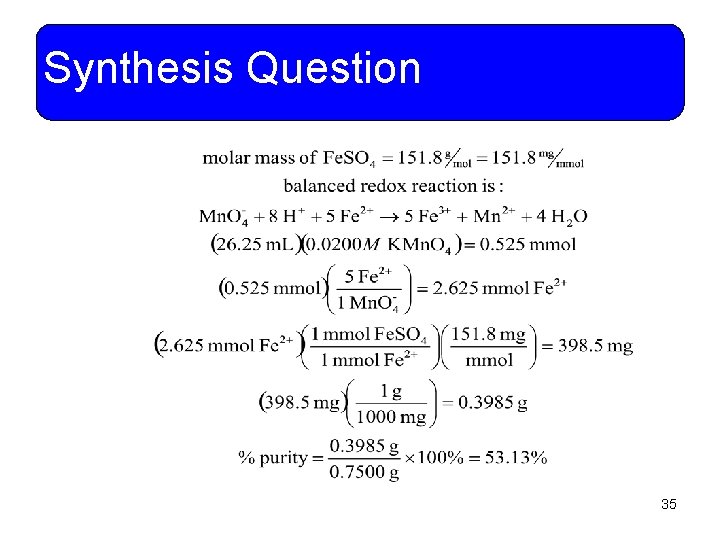
Synthesis Question • A 0. 7500 g sample of an impure Fe. SO 4 sample is titrated with 26. 25 m. L of 0. 0200 M KMn. O 4 to an endpoint. What is the % purity of the Fe. SO 4 sample? 34

Synthesis Question 35

11 Reactions in Aqueous Solutions II: Calculations 36