11 HEAT TRANSFER AND ENERGY BALANCES CHEMICAL ENGINEERING

11. HEAT TRANSFER AND ENERGY BALANCES CHEMICAL ENGINEERING 170
HEAT AND TEMPERATURE Temperature is a measure of how fast the atoms inside a system are moving The higher the temperature of something, the more kinetic energy there is inside of it. Heat is thermal energy that flows between systems. If you put something hot (high temperature) next to something cold (low temperature) Heat will flow from hot to cold How will the temperature change?
HEAT TRANSFER RATE Heat can flow at different speeds. Your skin senses heat transfer rate. Imagine: on a summer day, the inside of your car is 110°F Everything in the car is the same temperature So why does the metal seat belt buckle feel hotter than the cloth seat? Answer: the metal pours heat energy into your skin faster.
MODES OF HEAT TRANSFER Conduction: Heat transfer that comes from things at different temperature touching each other Good conductors (like metal) transfer heat more quickly than bad conductors (like wood). Gasses are usually very poor conductors of heat. Convection: Moving fluids carry heat Convection usually transfers heat more quickly than conduction (think wind on a cold day) Radiation: Heat transferred through electromagnetic waves Most obvious example is the sun, but everything with a temperature radiates heat!
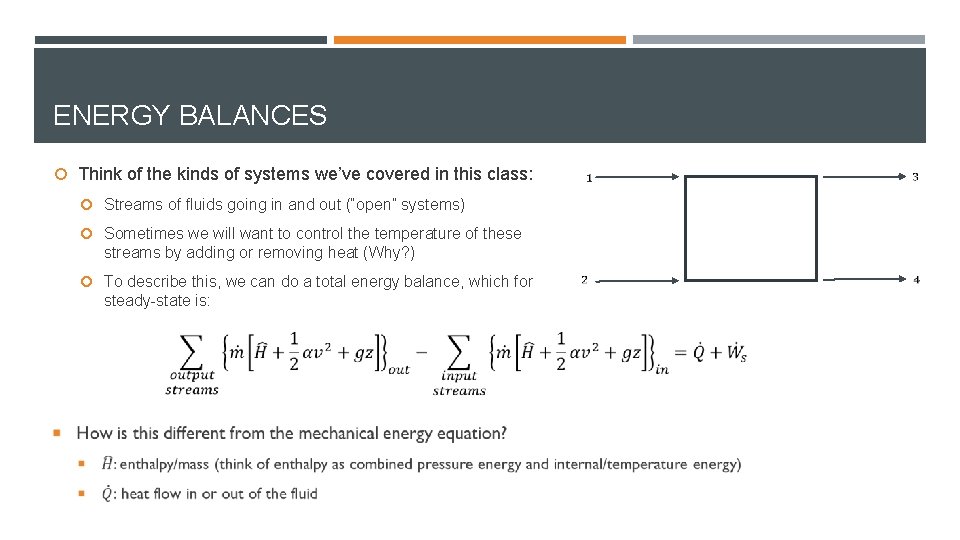
ENERGY BALANCES Think of the kinds of systems we’ve covered in this class: Streams of fluids going in and out (“open” systems) Sometimes we will want to control the temperature of these streams by adding or removing heat (Why? ) To describe this, we can do a total energy balance, which for steady-state is:
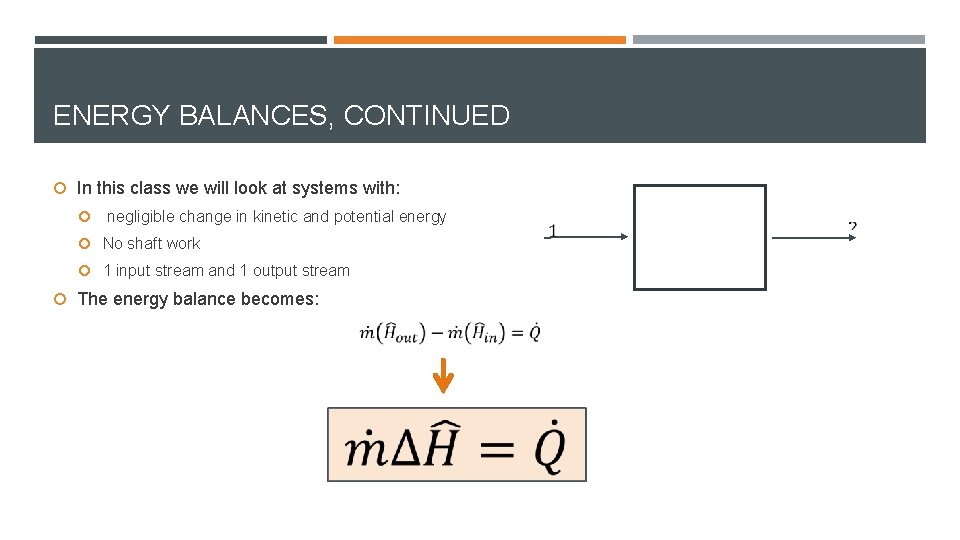
ENERGY BALANCES, CONTINUED In this class we will look at systems with: negligible change in kinetic and potential energy No shaft work 1 input stream and 1 output stream The energy balance becomes:
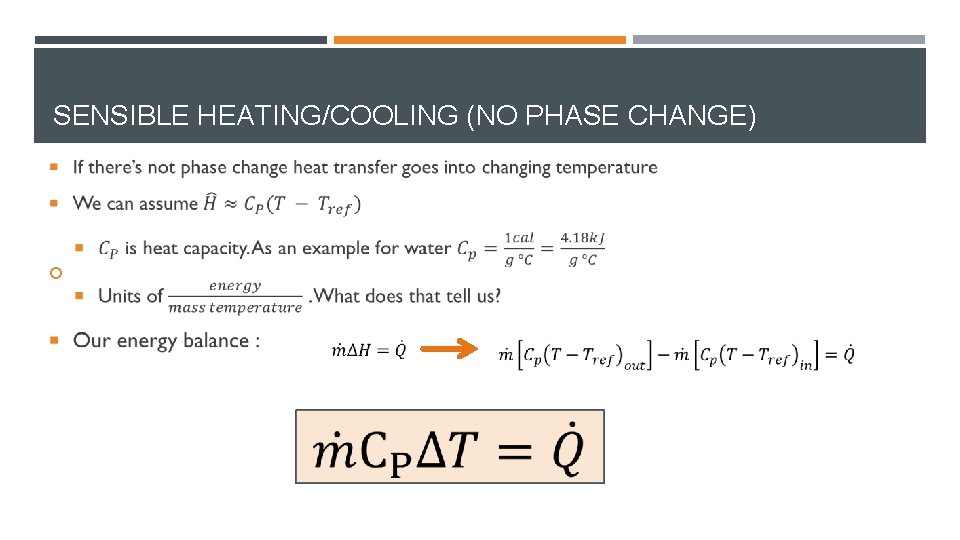
SENSIBLE HEATING/COOLING (NO PHASE CHANGE)
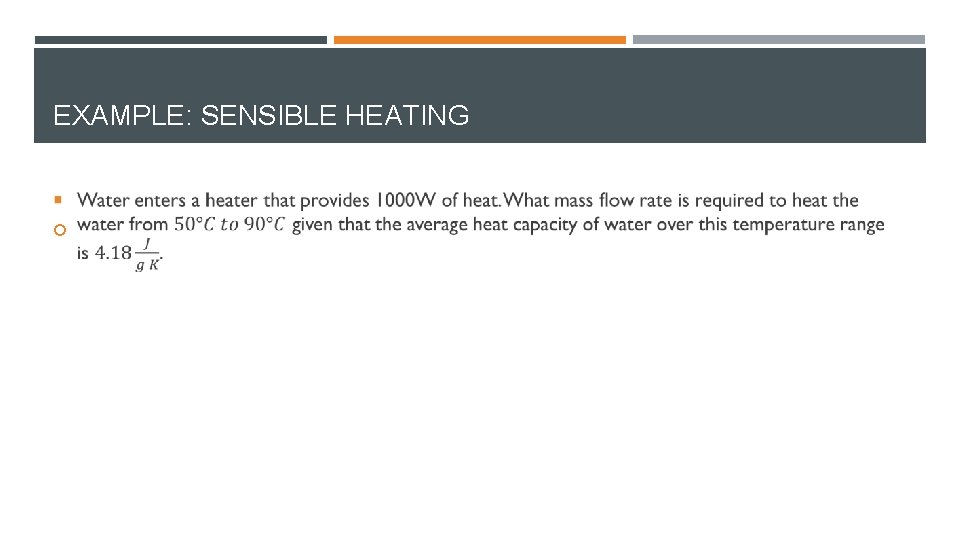
EXAMPLE: SENSIBLE HEATING
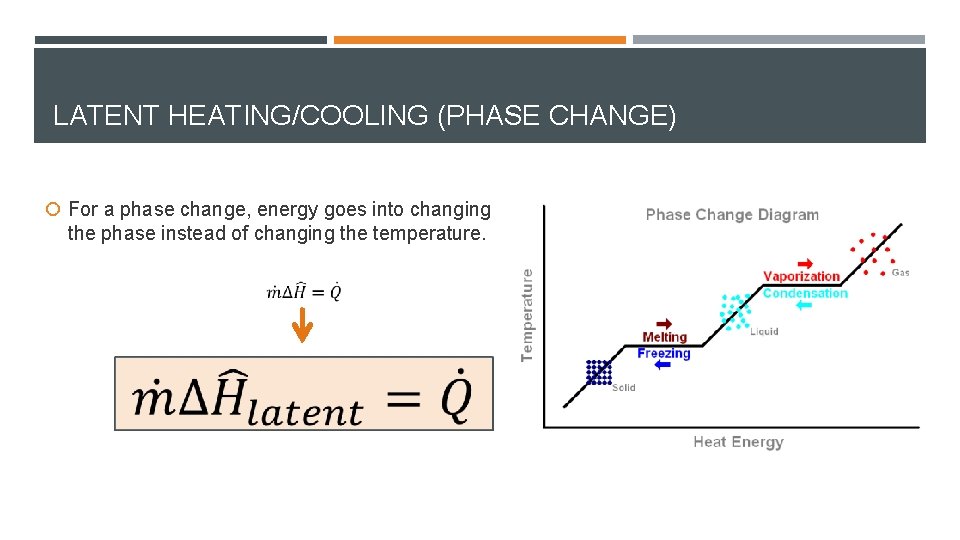
LATENT HEATING/COOLING (PHASE CHANGE) For a phase change, energy goes into changing the phase instead of changing the temperature.

LATENT HEATS
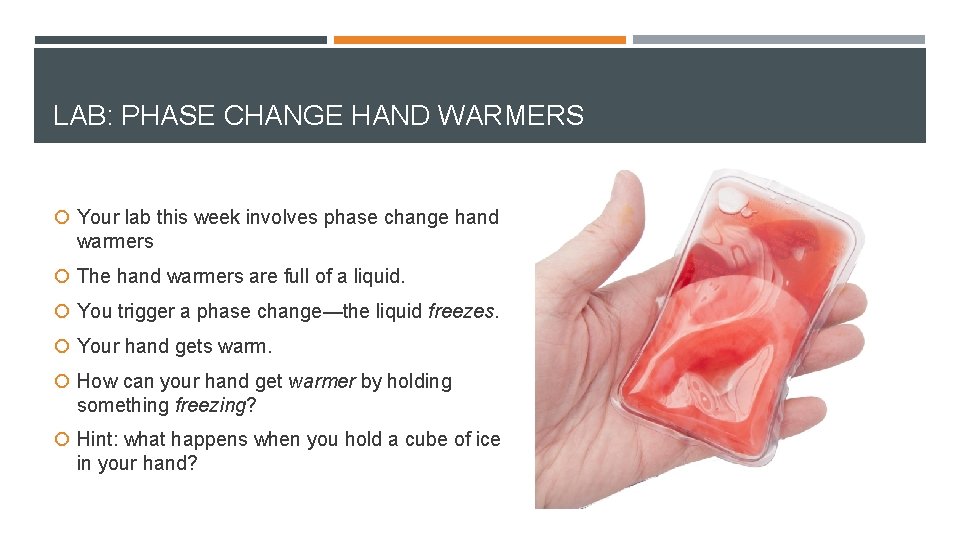
LAB: PHASE CHANGE HAND WARMERS Your lab this week involves phase change hand warmers The hand warmers are full of a liquid. You trigger a phase change—the liquid freezes. Your hand gets warm. How can your hand get warmer by holding something freezing? Hint: what happens when you hold a cube of ice in your hand?

MID-SEMESTER EVALUATION RESULTS Most of you think the pace of the class is about right But a substantial chunk think it’s too quick Comments requested more examples and review. I spend time on things like theory and where equations come from because I want you to understand. However, in the I will take a bit more for examples/review Especially because I want to have time for you to think about/work through examples yourself.

MID-SEMESTER EVALUATION RESULTS (CONTINUED…) I will add “unit hints” to some homework problems. A majority of you said a basic physics-review page would be necessary or helpful (will make this weekend) I apologize we took so long grading. From now on will be better. Multiple people said they felt afraid to ask questions in class. I promise it’s okay. You can always email me with questions outside of class.
- Slides: 13