11 Diamagnetism and Paramagnetism Ref Langevin Diamagnetism Equation

- Slides: 22

11. Diamagnetism and Paramagnetism • • • • Ref: Langevin Diamagnetism Equation Quantum Theory of Diamagnetism of Mononuclear Systems Paramagnetism Quantum Theory of Paramagnetism Rare Earth Ions Hund Rules Iron Group Ions Crystal Field Splitting Quenching of The Orbital Angular Momentum Spectroscopic Splitting Factor Van Vieck Temperature-Independent Paramagnetism Cooling by Isentropic Demagnetization Nuclear Demagnetization Paramagnetic Susceptibility of Conduction Electrons D. Wagner, “Introduction to the Theory of Magnetism”, Pergamon Press (72)

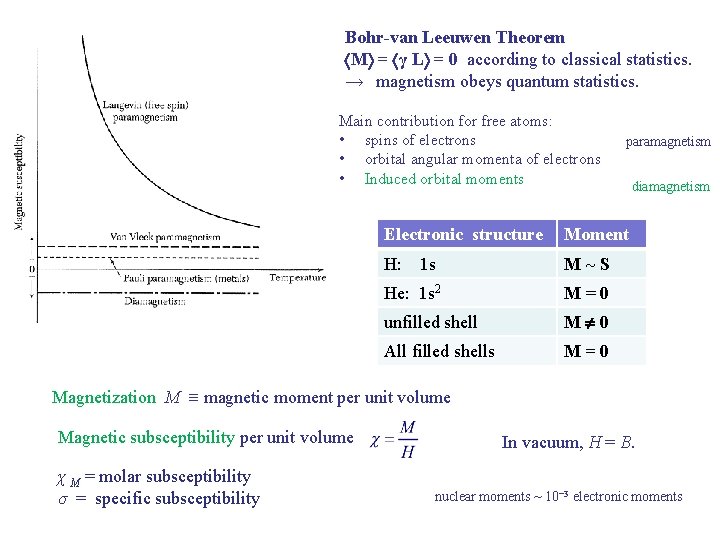
Bohr-van Leeuwen Theorem M = γ L = 0 according to classical statistics. → magnetism obeys quantum statistics. Main contribution for free atoms: • spins of electrons • orbital angular momenta of electrons • Induced orbital moments paramagnetism Electronic structure Moment H: 1 s M S He: 1 s 2 M=0 unfilled shell M 0 All filled shells M=0 diamagnetism Magnetization M magnetic moment per unit volume Magnetic subsceptibility per unit volume χ M = molar subsceptibility σ = specific subsceptibility In vacuum, H = B. nuclear moments ~ 10− 3 electronic moments

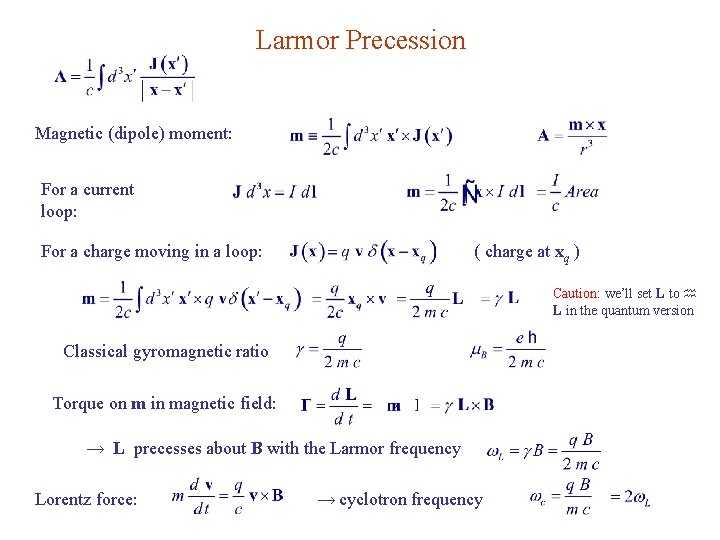
Larmor Precession Magnetic (dipole) moment: For a current loop: For a charge moving in a loop: ( charge at xq ) Caution: we’ll set L to L in the quantum version Classical gyromagnetic ratio Torque on m in magnetic field: → L precesses about B with the Larmor frequency Lorentz force: → cyclotron frequency

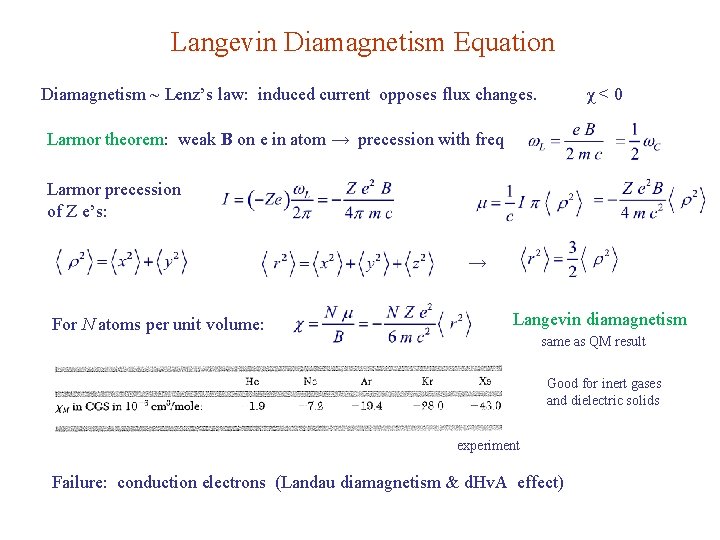
Langevin Diamagnetism Equation Diamagnetism ~ Lenz’s law: induced current opposes flux changes. χ<0 Larmor theorem: weak B on e in atom → precession with freq Larmor precession of Z e’s: → For N atoms per unit volume: Langevin diamagnetism same as QM result Good for inert gases and dielectric solids experiment Failure: conduction electrons (Landau diamagnetism & d. Hv. A effect)

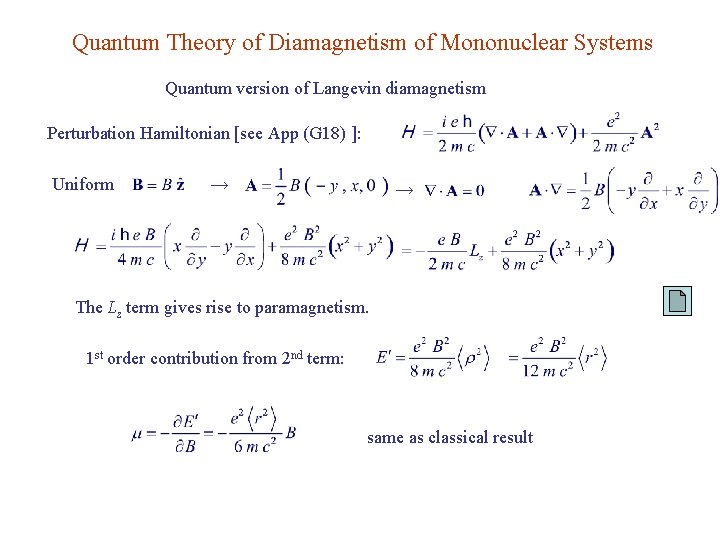
Quantum Theory of Diamagnetism of Mononuclear Systems Quantum version of Langevin diamagnetism Perturbation Hamiltonian [see App (G 18) ]: Uniform → → The Lz term gives rise to paramagnetism. 1 st order contribution from 2 nd term: same as classical result

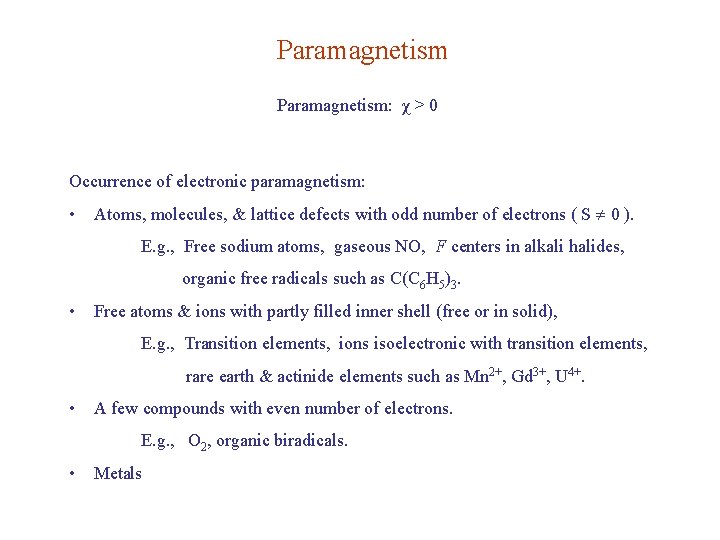
Paramagnetism: χ > 0 Occurrence of electronic paramagnetism: • Atoms, molecules, & lattice defects with odd number of electrons ( S 0 ). E. g. , Free sodium atoms, gaseous NO, F centers in alkali halides, organic free radicals such as C(C 6 H 5)3. • Free atoms & ions with partly filled inner shell (free or in solid), E. g. , Transition elements, ions isoelectronic with transition elements, rare earth & actinide elements such as Mn 2+, Gd 3+, U 4+. • A few compounds with even number of electrons. E. g. , O 2, organic biradicals. • Metals

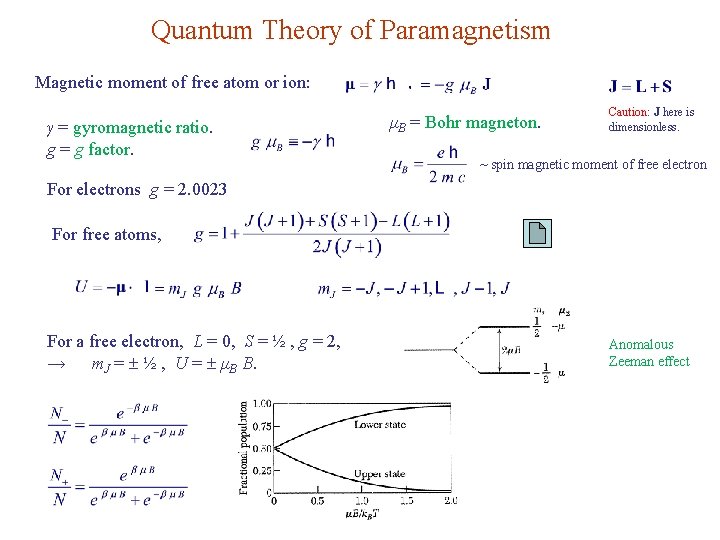
Quantum Theory of Paramagnetism Magnetic moment of free atom or ion: γ = gyromagnetic ratio. g = g factor. μB = Bohr magneton. Caution: J here is dimensionless. ~ spin magnetic moment of free electron For electrons g = 2. 0023 For free atoms, For a free electron, L = 0, S = ½ , g = 2, → m. J = ½ , U = μB B. Anomalous Zeeman effect

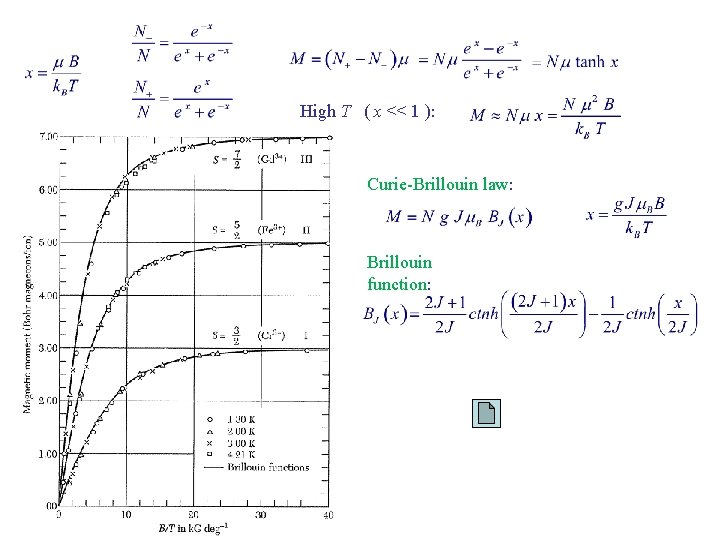
High T ( x << 1 ): Curie-Brillouin law: Brillouin function:

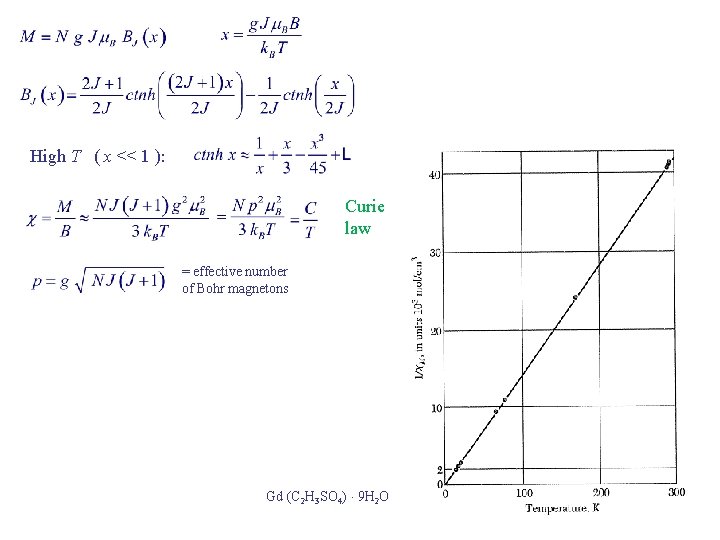
High T ( x << 1 ): Curie law = effective number of Bohr magnetons Gd (C 2 H 3 SO 4) 9 H 2 O

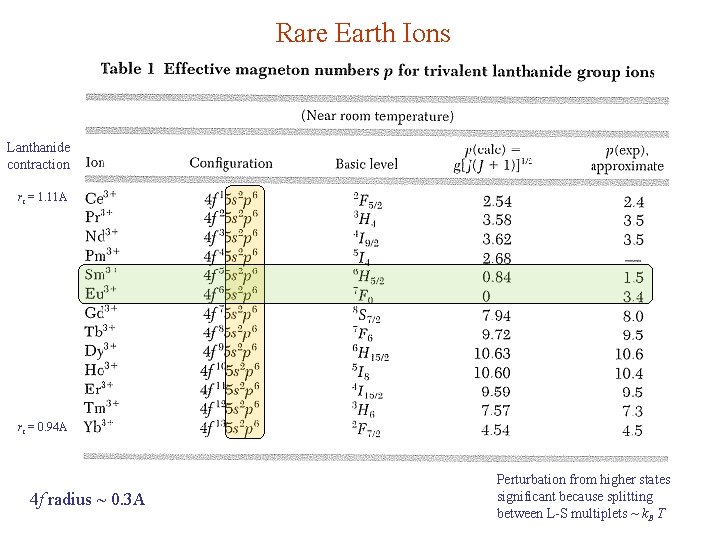
Rare Earth Ions Lanthanide contraction ri = 1. 11 A ri = 0. 94 A 4 f radius ~ 0. 3 A Perturbation from higher states significant because splitting between L-S multiplets ~ k. B T

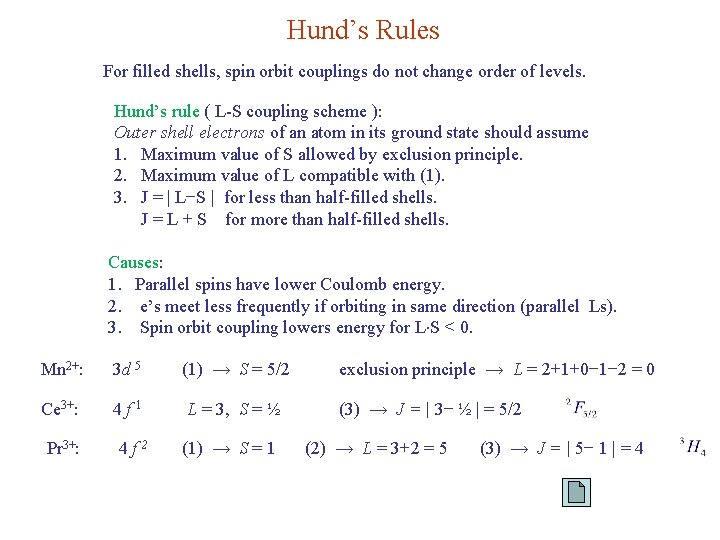
Hund’s Rules For filled shells, spin orbit couplings do not change order of levels. Hund’s rule ( L-S coupling scheme ): Outer shell electrons of an atom in its ground state should assume 1. Maximum value of S allowed by exclusion principle. 2. Maximum value of L compatible with (1). 3. J = | L−S | for less than half-filled shells. J = L + S for more than half-filled shells. Causes: 1. Parallel spins have lower Coulomb energy. 2. e’s meet less frequently if orbiting in same direction (parallel Ls). 3. Spin orbit coupling lowers energy for L S < 0. Mn 2+: 3 d 5 (1) → S = 5/2 Ce 3+: 4 f 1 L = 3, S = ½ Pr 3+: 4 f 2 (1) → S = 1 exclusion principle → L = 2+1+0− 1− 2 = 0 (3) → J = | 3− ½ | = 5/2 (2) → L = 3+2 = 5 (3) → J = | 5− 1 | = 4

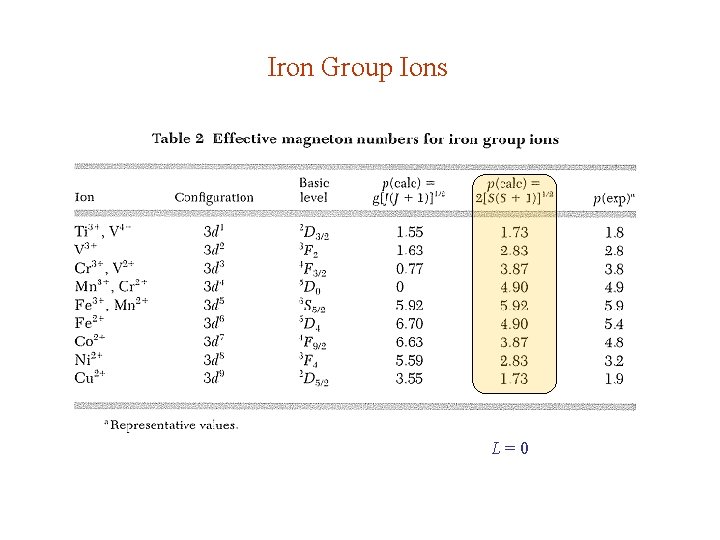
Iron Group Ions L=0

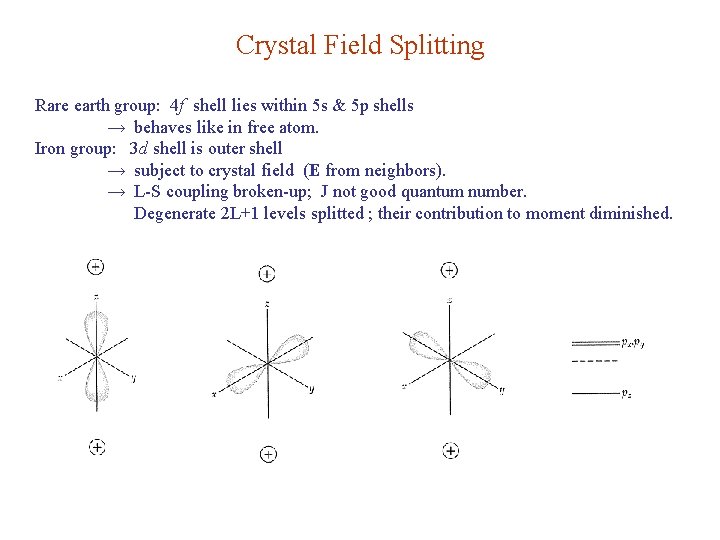
Crystal Field Splitting Rare earth group: 4 f shell lies within 5 s & 5 p shells → behaves like in free atom. Iron group: 3 d shell is outer shell → subject to crystal field (E from neighbors). → L-S coupling broken-up; J not good quantum number. Degenerate 2 L+1 levels splitted ; their contribution to moment diminished.

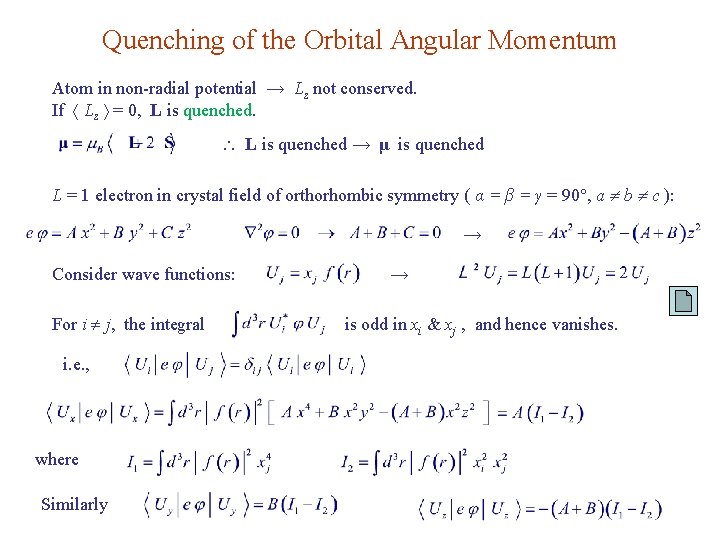
Quenching of the Orbital Angular Momentum Atom in non-radial potential → Lz not conserved. If Lz = 0, L is quenched → μ is quenched L = 1 electron in crystal field of orthorhombic symmetry ( α = β = γ = 90 , a b c ): → Consider wave functions: For i j, the integral i. e. , where Similarly → is odd in xi & xj , and hence vanishes.

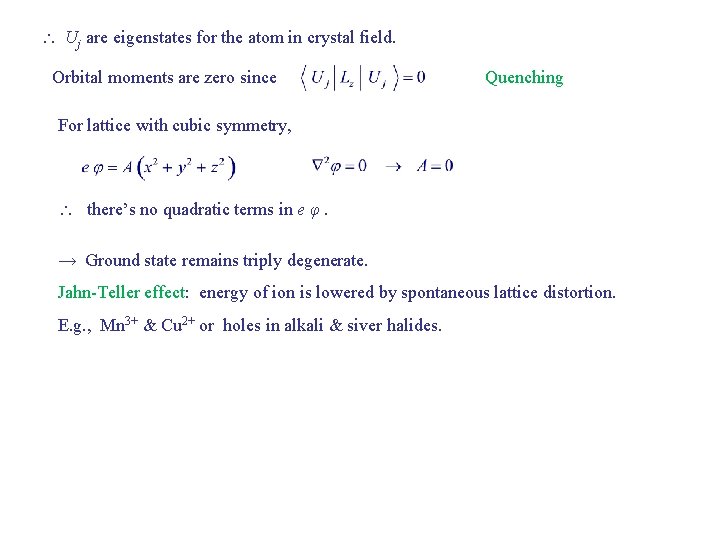
Uj are eigenstates for the atom in crystal field. Orbital moments are zero since Quenching For lattice with cubic symmetry, there’s no quadratic terms in e φ. → Ground state remains triply degenerate. Jahn-Teller effect: energy of ion is lowered by spontaneous lattice distortion. E. g. , Mn 3+ & Cu 2+ or holes in alkali & siver halides.

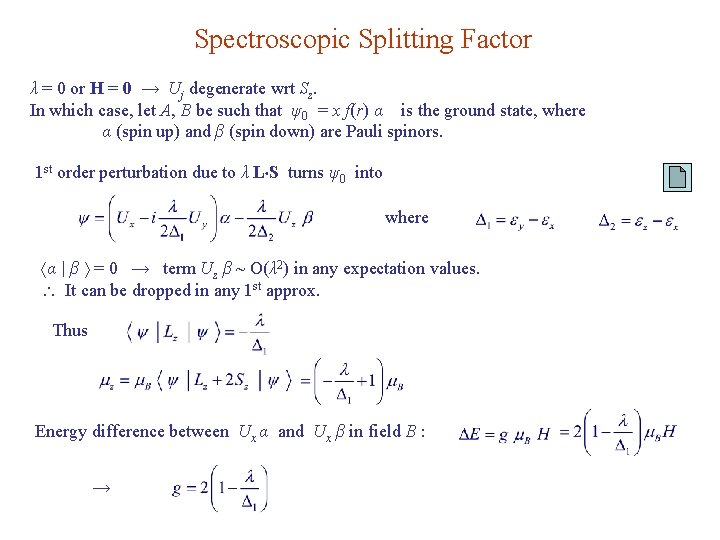
Spectroscopic Splitting Factor λ = 0 or H = 0 → Uj degenerate wrt Sz. In which case, let A, B be such that ψ0 = x f(r) α is the ground state, where α (spin up) and β (spin down) are Pauli spinors. 1 st order perturbation due to λ L S turns ψ0 into where α | β = 0 → term Uz β ~ O(λ 2) in any expectation values. It can be dropped in any 1 st approx. Thus Energy difference between Ux α and Ux β in field B : →

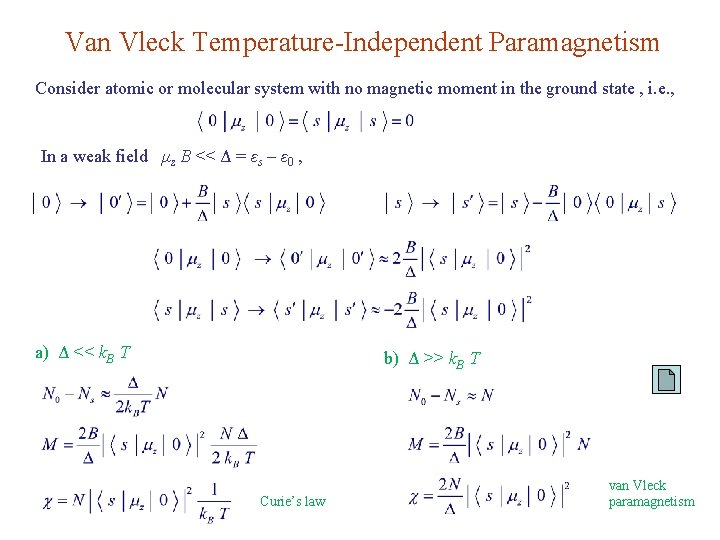
Van Vleck Temperature-Independent Paramagnetism Consider atomic or molecular system with no magnetic moment in the ground state , i. e. , In a weak field μz B << Δ = εs – ε 0 , a) Δ << k. B T b) Δ >> k. B T Curie’s law van Vleck paramagnetism

Cooling by Isentropic Demagnetization Was 1 st method used to achieve T < 1 K. Lowest limit ~ 10– 3 K. Mechanism: for a paramagnetic system at fixed T, Δ S < 0 as H increases. i. e. , H aligns μ and makes system more ordered. → Removing H isentropically (Δ S = 0) lowers T. Lattice entropy can seeps in during demagnetization Magnetic cooling is not cyclic.

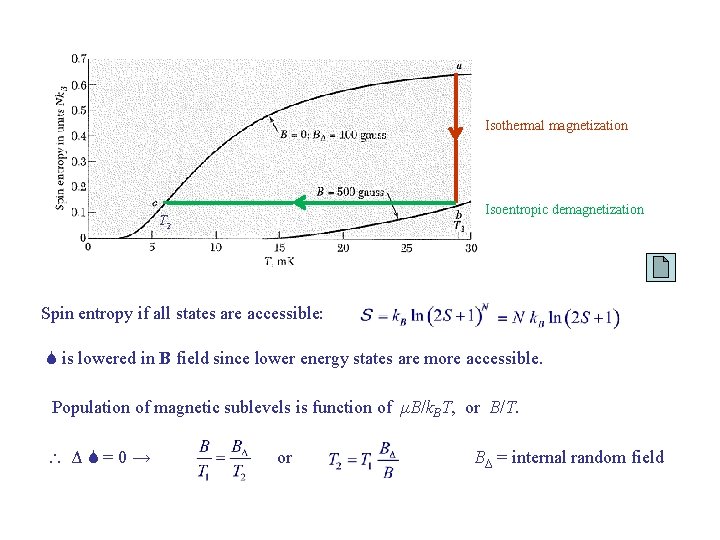
Isothermal magnetization Isoentropic demagnetization T 2 Spin entropy if all states are accessible: S is lowered in B field since lower energy states are more accessible. Population of magnetic sublevels is function of μB/k. BT, or B/T. ΔS=0→ or BΔ = internal random field

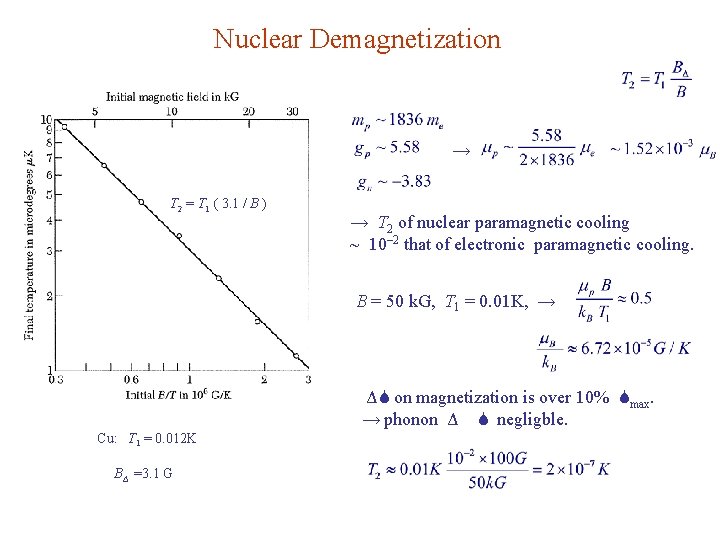
Nuclear Demagnetization → T 2 = T 1 ( 3. 1 / B ) → T 2 of nuclear paramagnetic cooling ~ 10– 2 that of electronic paramagnetic cooling. B = 50 k. G, T 1 = 0. 01 K, → ΔS on magnetization is over 10% Smax. → phonon Δ S negligble. Cu: T 1 = 0. 012 K BΔ =3. 1 G

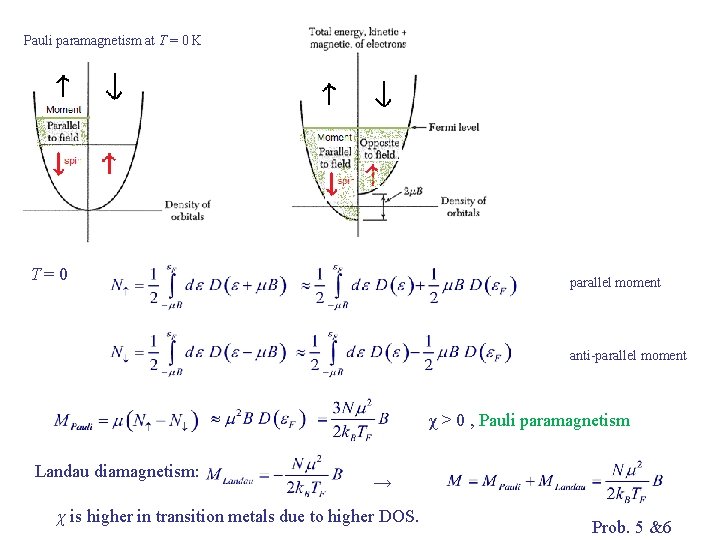
Paramagnetic Susceptibility of Conduction Electrons Classical free electrons: ~ Curie paramagnetism Experiments on normal non-ferromagnetic metals : M independent of T Pauli’s resolution: Electrons in Fermi sea cannot flip over due to exclusion principle. Only fraction T/TF near Fermi level can flip.

Pauli paramagnetism at T = 0 K T=0 parallel moment anti-parallel moment χ > 0 , Pauli paramagnetism Landau diamagnetism: → χ is higher in transition metals due to higher DOS. Prob. 5 &6