11 4 Electron Configurations And the Periodic Table

11. 4 Electron Configurations And the Periodic Table

11. 4 Electron Configurations (p 377) • • The electron configuration of hydrogen is “ 1 s 1” This means there is one electron in the 1 s orbital.
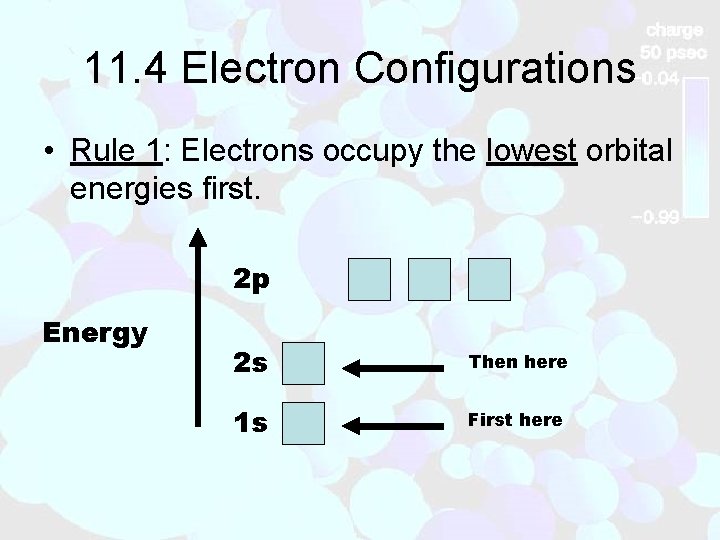
11. 4 Electron Configurations • Rule 1: Electrons occupy the lowest orbital energies first. 2 p Energy 2 s Then here 1 s First here
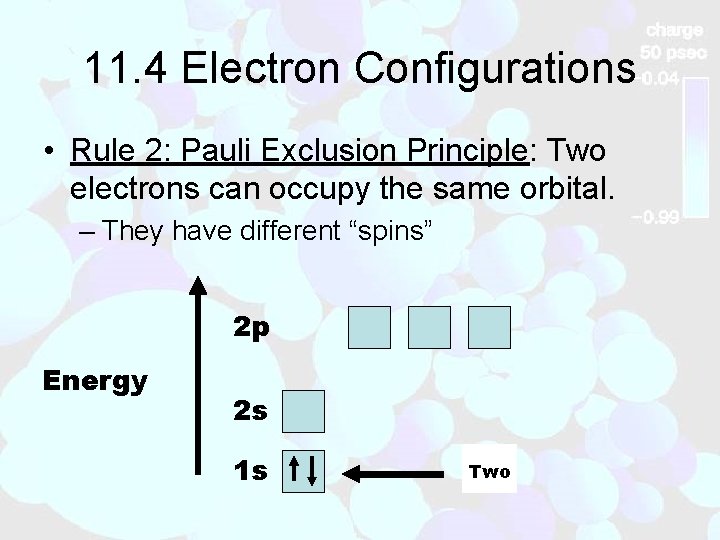
11. 4 Electron Configurations • Rule 2: Pauli Exclusion Principle: Two electrons can occupy the same orbital. – They have different “spins” 2 p Energy 2 s 1 s One Two
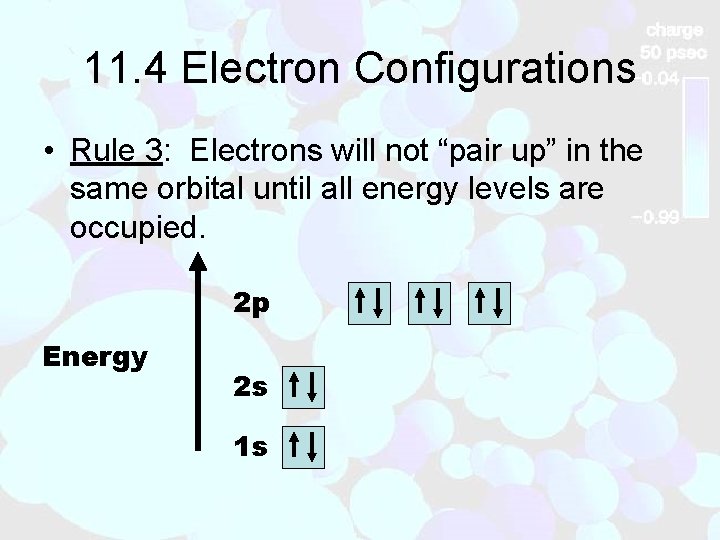
11. 4 Electron Configurations • Rule 3: Electrons will not “pair up” in the same orbital until all energy levels are occupied. 2 p Energy 2 s 1 s
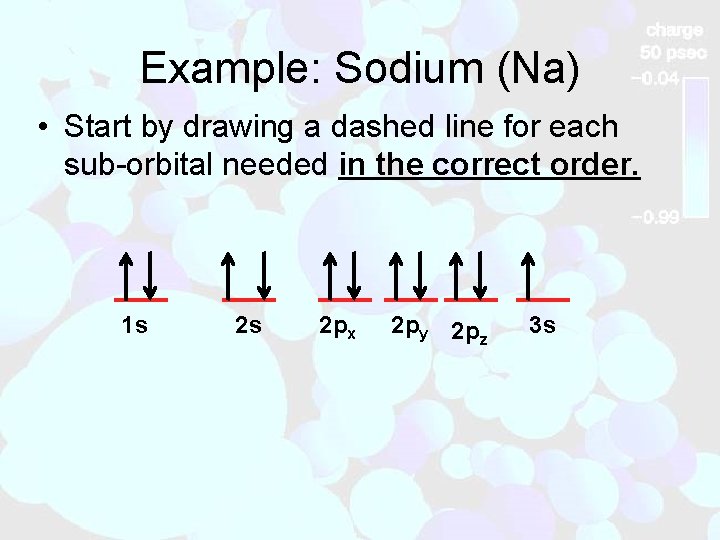
Example: Sodium (Na) • Start by drawing a dashed line for each sub-orbital needed in the correct order. 1 s 2 s 2 px 2 py 2 pz 3 s

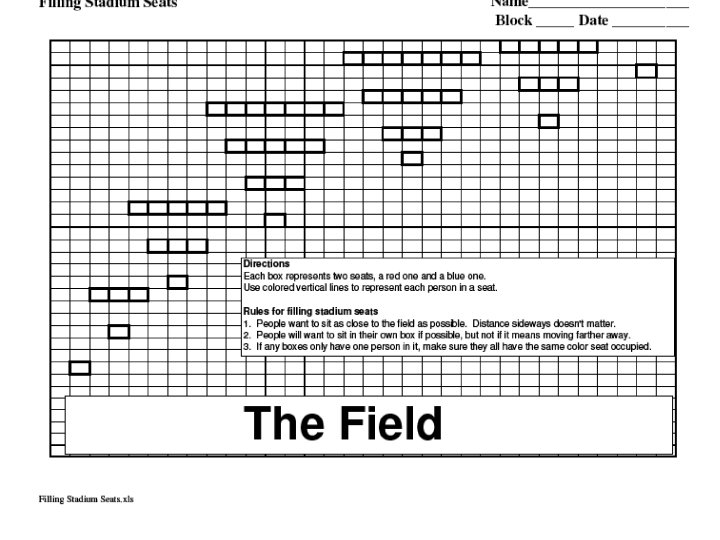
11. 4 Electron Configurations • Look at your field diagram • The order of the “seats” is the same as the order of orbitals. • Notice the 4 th energy level has seats closer than some of the 3 rd energy level. • Which orbital fills up first, 4 s or 3 d? – Yes, 3 d.
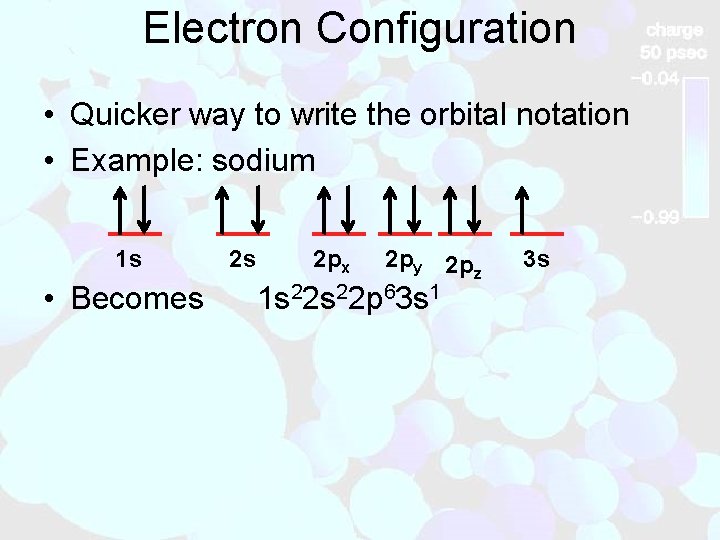
Electron Configuration • Quicker way to write the orbital notation • Example: sodium 1 s • Becomes 2 s 2 px 2 py 2 pz 1 s 22 p 63 s 1 3 s

11. 4 Electron Configurations • Examples: Li (Z=3), N (7), O(8) (See book) • Mg can be written as [Ne]3 s 2 • Valence electrons are the electrons in the highest principal energy level of an atom. They are involved in bonding with other atoms. • Nitrogen has electrons in n = 1 and 2. Level 2 is the valence level. 1 s 22 p 3 • Nitrogen has 5 valence electrons.

11. 4 Electron Configurations • • The core electrons are the inner electrons, and are not involved in bonding. Question: What elements have the same number of valence electrons as N? Where are they on the periodic table? Elements with the same number of valence electrons have similar chemical properties.
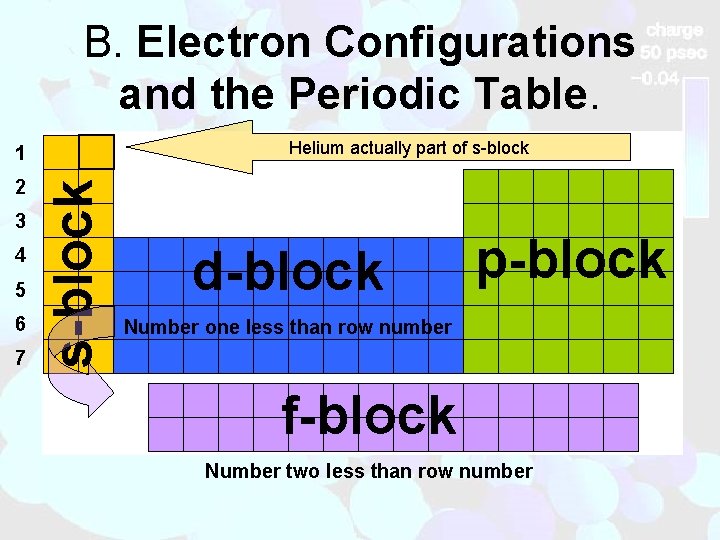
B. Electron Configurations and the Periodic Table. Helium actually part of s-block 2 3 4 5 6 7 s-block 1 d-block p-block Number one less than row number f-block Number two less than row number
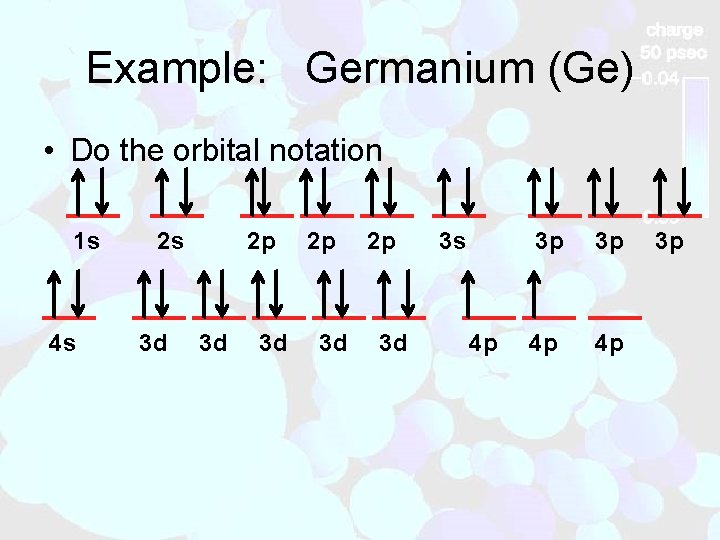
Example: Germanium (Ge) • Do the orbital notation 1 s 4 s 2 s 3 d 2 p 3 d 3 s 4 p 3 p 3 p 4 p 4 p 3 p
11. 4 C. Atomic Properties and the Periodic Table • Representative Elements - groups 1 A-8 A • Ionization – ionization is when an atom loses an electron. Metals • Remember: _______ lose electrons. Nonmetals • ________ gain electrons. • Going down a group, atoms are more likely to lose an electron. Cs is more likely to lose an electron than Li.
11. 4 C. Atomic Properties and the Periodic Table • Atomic size – decreases going up and right. • Decreases going up because electrons are closer at lower principal energy levels. • Decreases going right because the + charge in the nucleus is stronger.
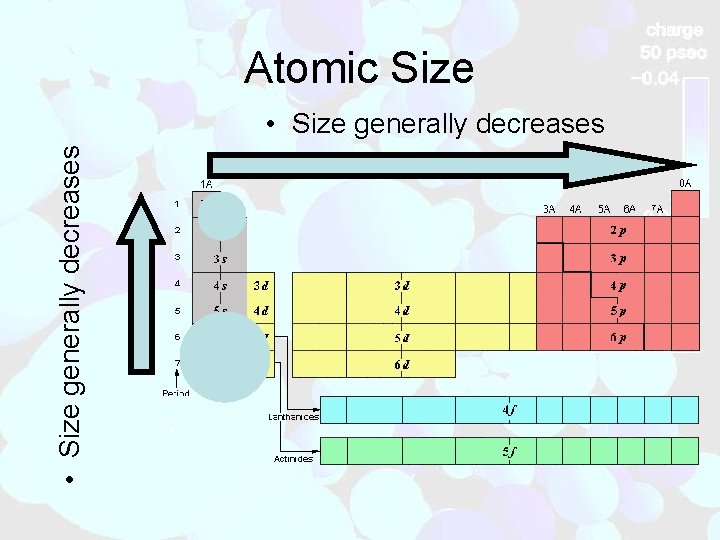
Atomic Size • Size generally decreases
11. 4 C. Atomic Properties and the Periodic Table • Ionization energy - the energy required to remove an electron from an atom in the gas phase. – increases up a group – increases to the right
Ionization Energy • Increases • increases

Checkpoint • Which element has a larger atomic size? – Sulfur or chlorine? – Answer: Sulfur • Which element has the larger ionization energy? – Sodium or potassium? – Answer: Sodium – http: //www. mhhe. com/physsci/chemistry/essentialche mistry/flash/atomic 4. swf – http: //video. google. com/videoplay? docid=2134266654801392897
- Slides: 19