11 2 Reaction Rate and Concentration YOU ARE
![Example 11. 1 • CH 3 CHO(g) CH 4(g) + CO(g) [ ] 0. Example 11. 1 • CH 3 CHO(g) CH 4(g) + CO(g) [ ] 0.](https://slidetodoc.com/presentation_image_h2/42767abae1e8ef2adc5bc77c7183de98/image-8.jpg)
![Calculate the Rate Constant • Rate = k[CH 3 CHO]2 • Choose one set Calculate the Rate Constant • Rate = k[CH 3 CHO]2 • Choose one set](https://slidetodoc.com/presentation_image_h2/42767abae1e8ef2adc5bc77c7183de98/image-9.jpg)


![Why Use a Logarithm? • If rate = k[A]m • Then ln rate = Why Use a Logarithm? • If rate = k[A]m • Then ln rate =](https://slidetodoc.com/presentation_image_h2/42767abae1e8ef2adc5bc77c7183de98/image-13.jpg)
- Slides: 14

11. 2 Reaction Rate and Concentration YOU ARE EXPECTED TO BE ABLE TO: • Define the initial rate of a reaction as the instantaneous rate at the start of the reaction. • Write a rate law expression for a given reaction given experimental results that show the variation of initial rate with concentration. • Define the concept of order of reaction and determine the order of a given reaction from initial rate vs concentration data Chemistry 1011 Slot 5 1

Initial Rate of Reaction • The initial rate of reaction is the instantaneous rate measured at the start of the reaction • The initial rate can be used as a benchmark • Measurements of the initial rate for a reaction at different starting concentrations can be used to determine the effect of concentration on rate Chemistry 1011 Slot 5 2

The Rate Expression or Rate Law • In general: – Reactions occur as a result of collisions – The higher the concentration of molecules, the greater the rate • In some reactions, the rate is directly proportional to the concentration • In other cases, the rate may increase much more quickly than the concentration • The relationship between rate and concentration is known at the rate law or rate expression Chemistry 1011 Slot 5 3

Determining the Rate Law Expression • The rate law MUST be determined from experiment – it is not related to the stoichiometry of the equation for the reaction • Examine the concentration vs time graph for N 2 O 5 – Determine instantaneous rates at different times and concentrations of N 2 O 5 – Plot instantaneous rate vs [N 2 O 5 ] – This is a straight line, (equation y = mx + b) • Rate = k[N 2 O 5] ; this is the rate law expression; k is the rate constant Chemistry 1011 Slot 5 4

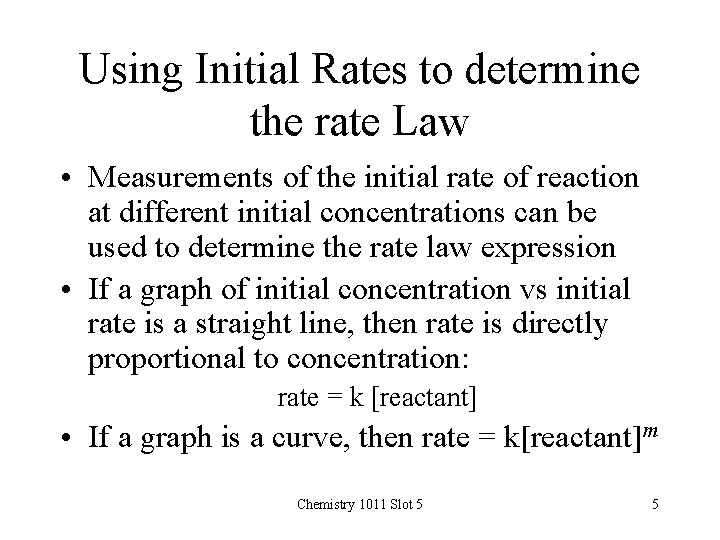
Using Initial Rates to determine the rate Law • Measurements of the initial rate of reaction at different initial concentrations can be used to determine the rate law expression • If a graph of initial concentration vs initial rate is a straight line, then rate is directly proportional to concentration: rate = k [reactant] • If a graph is a curve, then rate = k[reactant]m Chemistry 1011 Slot 5 5

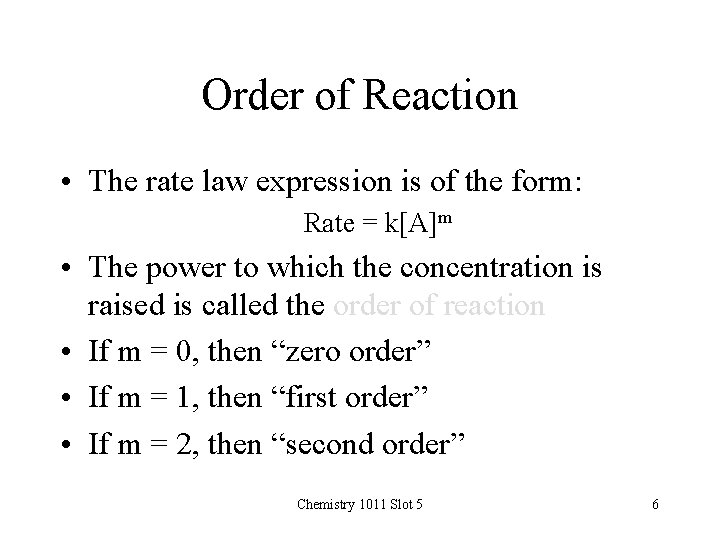
Order of Reaction • The rate law expression is of the form: Rate = k[A]m • The power to which the concentration is raised is called the order of reaction • If m = 0, then “zero order” • If m = 1, then “first order” • If m = 2, then “second order” Chemistry 1011 Slot 5 6

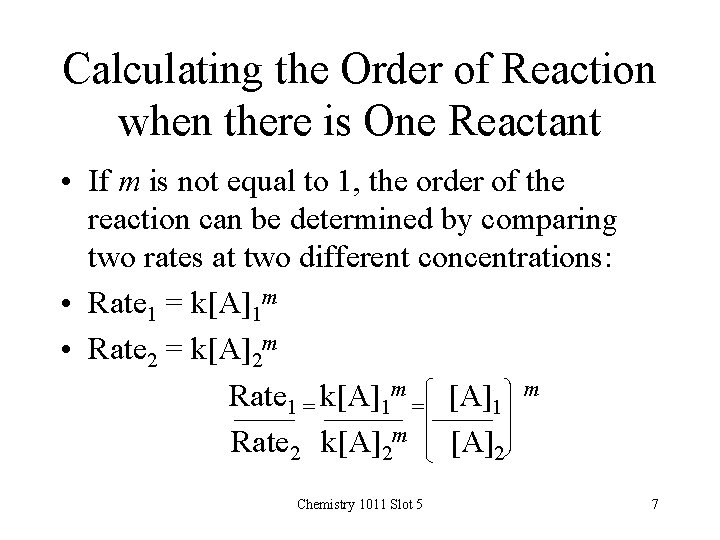
Calculating the Order of Reaction when there is One Reactant • If m is not equal to 1, the order of the reaction can be determined by comparing two rates at two different concentrations: • Rate 1 = k[A]1 m • Rate 2 = k[A]2 m Rate 1 = k[A]1 m = [A]1 m Rate 2 k[A]2 m [A]2 Chemistry 1011 Slot 5 7
![Example 11 1 CH 3 CHOg CH 4g COg 0 Example 11. 1 • CH 3 CHO(g) CH 4(g) + CO(g) [ ] 0.](https://slidetodoc.com/presentation_image_h2/42767abae1e8ef2adc5bc77c7183de98/image-8.jpg)
Example 11. 1 • CH 3 CHO(g) CH 4(g) + CO(g) [ ] 0. 10 M 0. 20 M 0. 30 M Rate (mol/L. s) 0. 085 0. 34 0. 76 • Choose the first two concentrations 0. 40 M 1. 4 Rate 2 = 0. 34 = 4. 0 Rate 1 0. 085 [CH 3 CHO]2 = 0. 20 = 2. 0 [CH 3 CHO]1 0. 10 4. 0 = (2. 0)m ; m = 2 Rate = k[CH 3 CHO]2 The reaction is second order Chemistry 1011 Slot 5 8
![Calculate the Rate Constant Rate kCH 3 CHO2 Choose one set Calculate the Rate Constant • Rate = k[CH 3 CHO]2 • Choose one set](https://slidetodoc.com/presentation_image_h2/42767abae1e8ef2adc5bc77c7183de98/image-9.jpg)
Calculate the Rate Constant • Rate = k[CH 3 CHO]2 • Choose one set of data 0. 085 mol/L. s = k x (0. 10 mol/L)2 k = 0. 085 mol/L. s = 8. 5 L/mol. s (0. 10 mol/L)2 Chemistry 1011 Slot 5 9

Order of Reaction with More Than One Reactant • Most reactions involve >1 reactant a. A + b. B products • General rate law expression is: rate = k[A]m x [B]n – m is “order with respect to A – n is order with respect to B – Overall order is m + n Chemistry 1011 Slot 5 10

Determining the Order • Hold concentration of one reactant constant and vary other - measure initial rate • Repeat, holding concentration of other reactant constant • See Example 11. 2, page 308 Chemistry 1011 Slot 5 11

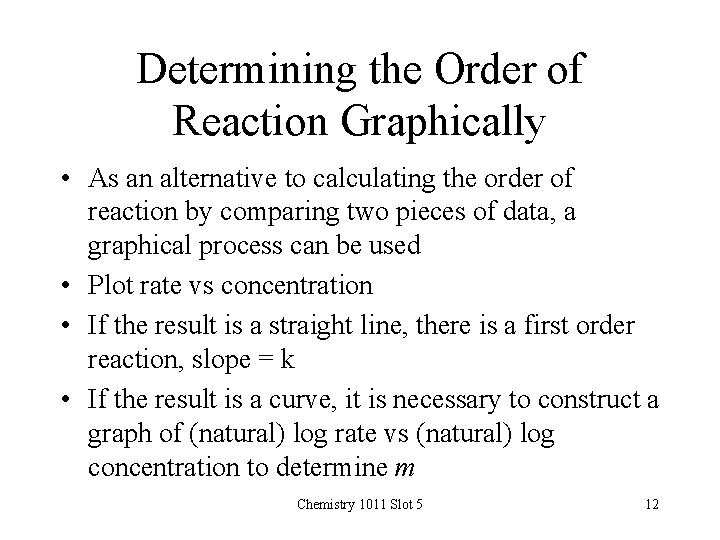
Determining the Order of Reaction Graphically • As an alternative to calculating the order of reaction by comparing two pieces of data, a graphical process can be used • Plot rate vs concentration • If the result is a straight line, there is a first order reaction, slope = k • If the result is a curve, it is necessary to construct a graph of (natural) log rate vs (natural) log concentration to determine m Chemistry 1011 Slot 5 12
![Why Use a Logarithm If rate kAm Then ln rate Why Use a Logarithm? • If rate = k[A]m • Then ln rate =](https://slidetodoc.com/presentation_image_h2/42767abae1e8ef2adc5bc77c7183de98/image-13.jpg)
Why Use a Logarithm? • If rate = k[A]m • Then ln rate = ln k + m ln [a] • This will be a straight line with slope m Chemistry 1011 Slot 5 13

Zero Order Reactions • In rare cases, the rate of reaction is independent of the concentration of reactant(s) • In these cases – Rate = k[A]0 • A plot of rate vs [A] would be a horizontal straight line Chemistry 1011 Slot 5 14