11 1 Introduction 1 When separation by distillation

§ 11. 1 Introduction 1 When separation by distillation is ineffective or very difficult, liquid extraction is one of the main alternatives to consider. 即:先Distillation,再 Liquid Extraction 2 Close-boiling mixtures or substances that cannot withstand the temperature of distillation even under a vacuum may often be separated from impurities by extraction, which utilize chemical differences instead of vapor pressure differences. ~ distillation principle 什么情况下才考虑用Extraction?
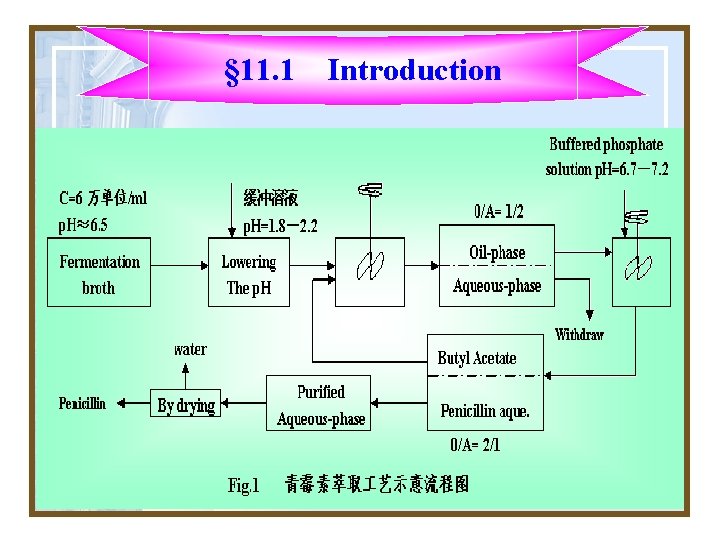
§ 11. 1 Introduction 3. For example Penicillin is recovered from the fermentation broth by extraction with a solvent such as butyl acetate (丁 醋酸,有机物,比水轻), after lowering the p. H to a favorable partition coefficient(分割系数).

§ 11. 1 Introduction 4. The solvent is then treated with a buffered phosphate solution(磷酸缓冲液)to extract the penicillin from the solvent and give a purified aqueous solution, from which penicillin is eventually produced by drying.
§ 11. 1 Introduction



§ 11. 1 Introduction 5. Extraction is also used to recover acetic acid(醋酸) from dilute aqueous solutions ;distillation would be possible in this case ,but the extraction step considerably reduces(大大减少)the amount of water to be distilled(所需要蒸馏的水量). Extraction benefit

§ 11. 1 Introduction 6. One of the major uses of extraction is to separate petroleum products that have different chemical structures but about the same boiling range(沸程几乎相同).

§ 11. 1 Introduction 7. Lube oil fractions(润滑油馏分)(Bp>300℃) are treated with low-boiling-point polar solvents such as phenol(石碳酸), furfural(糠醛)or methyl-pyrrolidone(甲基四氢吡咯烷酮)to extract the aromatics(芳烃)and leave an oil that contains mostly paraffins(链烷烃)and naphthenes(环烷烃).

§ 11. 1 Introduction 8. The aromatics have poor viscositytemperature characteristics, but they cannot be removed by distillation because of the overlapping boiling-point ranges( 沸程重叠).

§ 11. 1 Introduction 9. In a similar process, aromatics are extracted from catalytic reformats(催化重整产物)using a highboiling-point polar solvent, and the extract(萃取 物)is later distilled to give pure benzene, toluene, and xylenes(二甲苯:邻,间,对)for use as chemical intermediates(化学中间体). (Aromatics ~ 简称BTX)

§ 11. 1 Introduction 10. An excellent solvent for this use is the cyclic compound(杂环化合物)C 11 H 8 SO 2 (Sulfolane)(四氢噻吩砜,环丁砜) which has high selectivity for aromatics and very low volatility(Bp of 290℃).

§ 11. 1 Introduction 11. When either distillation or extraction may be used, the choice is usually distillation, in spite of the fact that heating and cooling are needed. Why? The reason 12. In extraction the solvent must be recovered for reuse (usually by distillation), and the combined operation(组合操作)is more complicated and expensive than ordinary distillation without extract. ~disadvantage

§ 11. 1 Introduction 13. However, extraction does offer greater flexibility in choice of operating conditions, since the type and amount of solvent can be varied as well as the operating temperature. Benefits

§ 11. 1 Introduction 14. In this sense, extraction is more like gas absorption than ordinary distillation. In many problems, the choice between methods should be based on a comparative study(比较)of both extraction and distillation.

§ 11. 1 Introduction 15. Extraction maybe used to separate more than two components; and mixtures of solvents, (萃取剂也可以是混合物) instead of a single solvent, are needed in some application. Those more complicated methods are not treated in this text.
- Slides: 16