108 Remember the electron cloud model Instead of
10/8

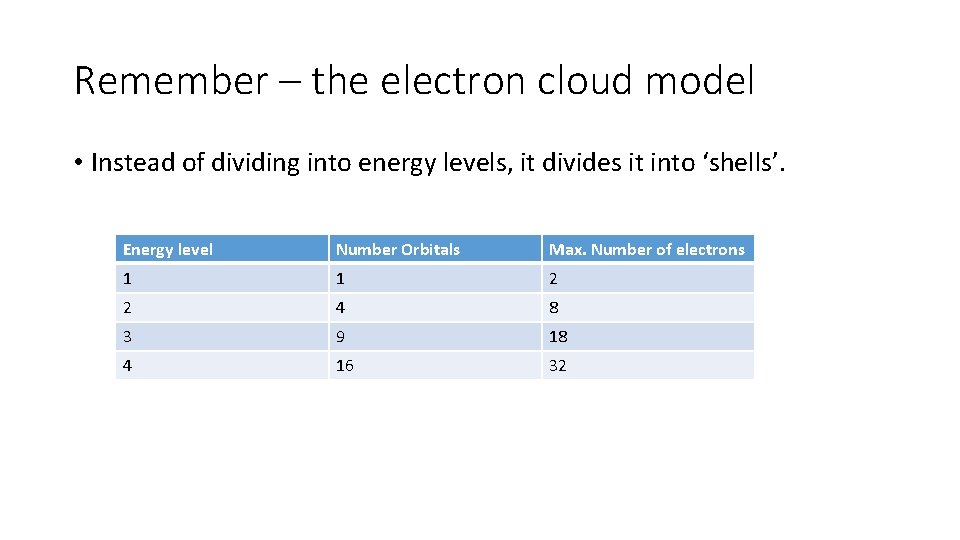
Remember – the electron cloud model • Instead of dividing into energy levels, it divides it into ‘shells’. Energy level Number Orbitals Max. Number of electrons 1 1 2 2 4 8 3 9 18 4 16 32

Periodic table • Useful to group elements based on properties and arranged by increasing atomic numbers • Period – horizontal row • Group – vertical coloumn
Periods • In a period, atomic number and number of energy levels increases from left to right

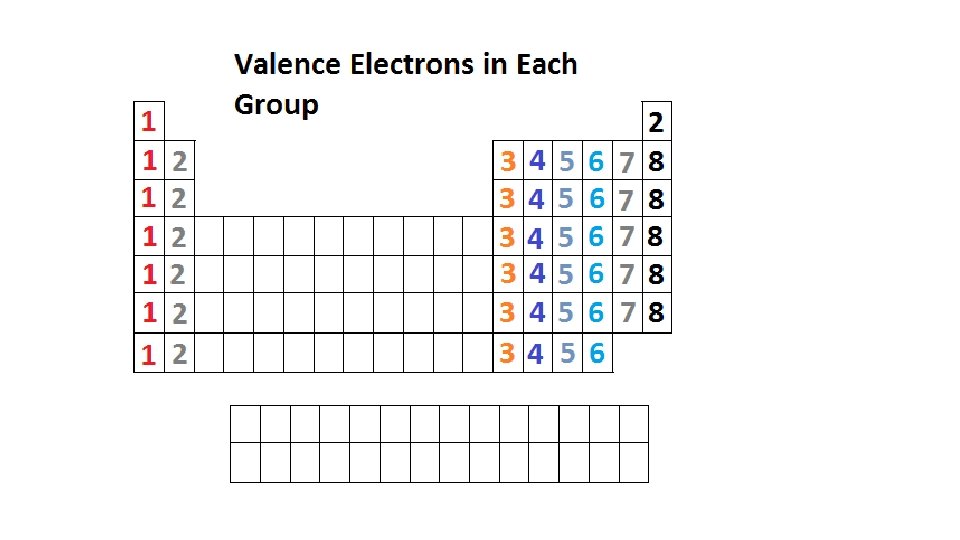
Groups • Within a group, elements have the same properties because of similar electron configurations – they have the same number of valence electrons • Periodic law – pattern of repeating properties

The Alkali Metals – Group 1 A • One valence electron • Very reactive – found only in compounds (Na. Cl) • Solids at room temperature • Low Density • Relatively soft

The Alkaline Earth Metals – Group 2 A • 2 valence electrons • Very reactive (not as reactive as alkali metals) • Reactivity increases from top to bottom • Silver or gray solids at room temperature • Soft (not as soft as alkali metals) • Low density • Calcium and Magnesium have biological importance

Transition Metals – B groups • Variable valence electrons • Less reactive than groups 1 A and 2 A • Metals • Hard, shiny solids • Superior ability to conduct electricity and heat • High melting and boiling points

The Boron Family – Group 3 A • 3 valence electrons • Boron – metalloid, the rest are metals • Less reactive than Group 1 A or Group 2 A • Aluminum is very abundant, but gallium, indium and thallium are rare

The Carbon Family – Group 4 A • 4 valence electrons • Not very reactive – can exist on their own • Solids at room temperature • Metallic nature increases from top to botton • Carbon is a non metal • Silicon and germanium are metalloids • Tin and lead are metals • Carbon very important for life on Earth • Silicon second most abundant element on Earth
The Nitrogen Family – Group 5 A • 5 valence electrons • Not very reactive – can exist on their own • Metallic nature increases from top to bottom • Nitrogen nonmetal gas and phosphorous nonmetal solid • Arsenic and antimony solid metalloids • Bismuth solid metal • Nitrogen and phosphorous are used in fertilizer
The Oxygen Family – Group 6 A • 6 valence electrons • Reactive • Except oxygen, all are solid at room temperature • Metallic character increases down the table • Oxygen, sulfur and selenium are nonmetals • Tellurium and polonium are metals • Oxygen is the most abundant element on Earth

Halogens – Group 7 A • 7 valence electrons • Very reactive – especially with most metals • Reactivity increases down the table • Non metals • Vary in state (solid, gas) and color • Fluorine prevents tooth decay, chlorine kills bacteria, iodine is needed for your thyroid gland

The Noble Gases – Group 8 A • 8 valence electrons, except Helium which as 2 • Extremely unreactive • Colorless • Odorless • Gases • All of the noble gases, except radon, are used for “neon” signs.

Metals, non-metals and metalloids • Metals are good conductors of heat • Non metals poor conductors of heat and electricity • Metalloids the ability to conduct heat and electricity vary with heat.

Guided Practice Alkali Metals have 1 valence electrons. They are very reactive and do not exist on their own. They are solids at room temperature and have low density.
- Slides: 18