1027 DO NOW Hmmm Write down your thoughts




















- Slides: 41

10/27 DO NOW Hmmm – Write down your thoughts to these open ended questions: 1. How can we know what's in an atom if we can't even see one? 2. Is there anything smaller than protons, neutrons and electrons? Explain.

AGENDA 10/27 1. 2. 3. 4. 5. Do Now Go over test HW on the website Handouts Begin Chapter 4 (if time) HW: None

10/27 DO NOW 1. If you were in the history/science books for having invented or discovered something, what would you want to have discovered/invented? 2. What materials or equipment would you need for this discovery/invention? 3. Explain the earliest steps you’d take in this endeavor. (Hint: What things would you need to do at the very beginning of your research/experimentation? )

The Structure of the Atom CHAPTER 4

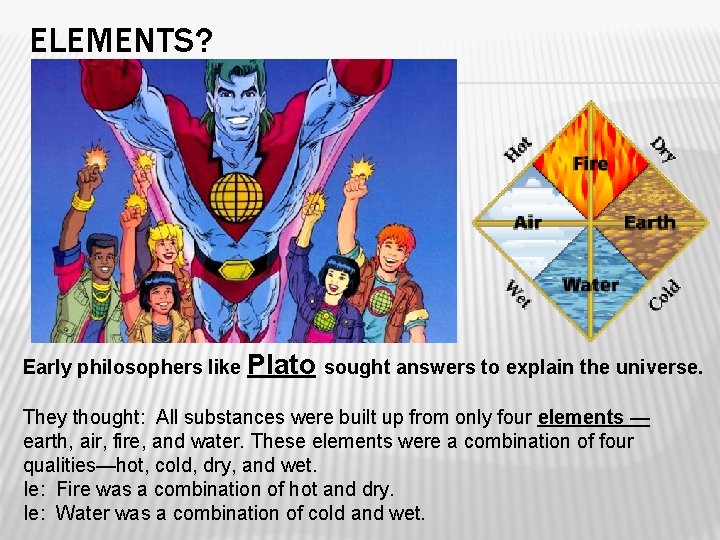
ELEMENTS? Early philosophers like Plato sought answers to explain the universe. They thought: All substances were built up from only four elements — earth, air, fire, and water. These elements were a combination of four qualities—hot, cold, dry, and wet. Ie: Fire was a combination of hot and dry. Ie: Water was a combination of cold and wet.

10/30 1. AGENDA Begin Chapter 4: Atomic Structure - History of Atomic Discovery 2. 3. 4. “Draw the Atom” Timeline Exit Ticket HW Packet pgs 2 -3

4. 1 STUDYING ATOMS � � � If you cut a piece of Aluminum foil in half and continue to cut the resulting piece in half, what will happen? Are we slicing an atom in half? ? Greek Philosophers pondered this 2500 years ago. � Democritus- believed all matter consisted of extremely small particles that could not be divided. � He � called these particles atoms Aristotle – did not think there was a “Atomos” limit to the number of times matter could be divided. � Most people believed Aristotle until the 1800 s when scientists had enough data to support Democritus

MYSTERY OF THE ATOM Lots of people tried to figure out: 1. What the atom looked like 2. How atoms behave

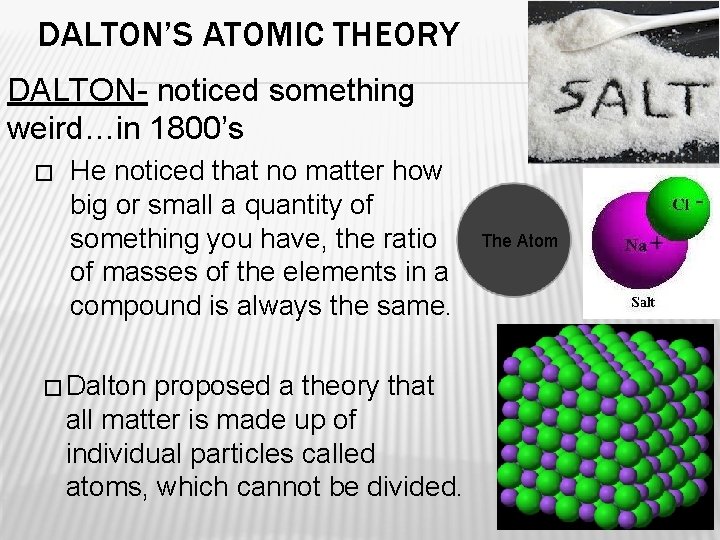
DALTON’S ATOMIC THEORY DALTON- noticed something weird…in 1800’s � He noticed that no matter how big or small a quantity of something you have, the ratio of masses of the elements in a compound is always the same. � Dalton proposed a theory that all matter is made up of individual particles called atoms, which cannot be divided. The Atom

MAIN POINTS OF DALTON’S ATOMIC THEORY 1. 2. 3. All elements are composed of atoms. All atoms of the same element have the same mass, which are unique from other elements. Compounds contain more than 1 element. Note: This isn’t news! We already learned these above!

MAIN POINTS OF DALTON’S ATOMIC THEORY 4. In a compound, atoms will always combine the same way. 5. Dalton thought elements were solid spheres. Each type of atom is represented by a tiny, solid sphere with a different mass. ***Eventually, scientists discovered not all of Dalton’s theories were correct… Note: This STILL isn’t news!

THOMSON’S MODEL OF THE ATOM J. J. Thomson- 1856 -1940 � Atoms have positive and negative charges. � Objects with like charges repel, or push apart. � Objects with opposite charges attract or pull together. � Some charged particles can flow from one location to another (electric current) � Thomson used an electric current to learn more about atoms. 0 + -

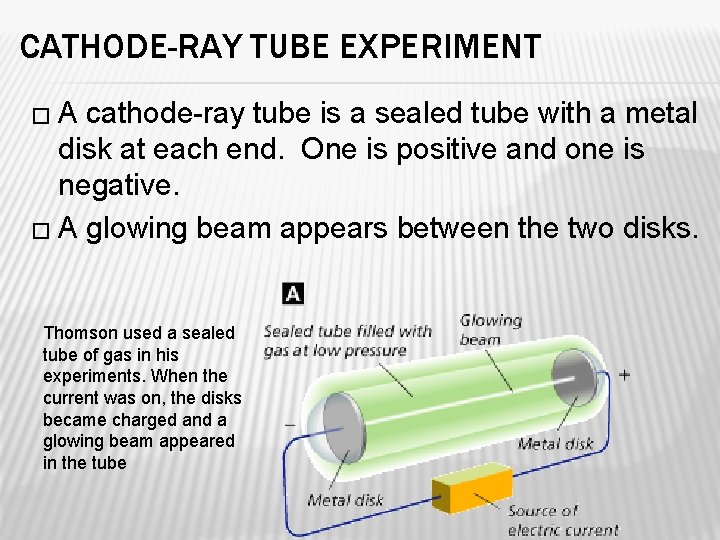
CATHODE-RAY TUBE EXPERIMENT �A cathode-ray tube is a sealed tube with a metal disk at each end. One is positive and one is negative. � A glowing beam appears between the two disks. Thomson used a sealed tube of gas in his experiments. When the current was on, the disks became charged and a glowing beam appeared in the tube

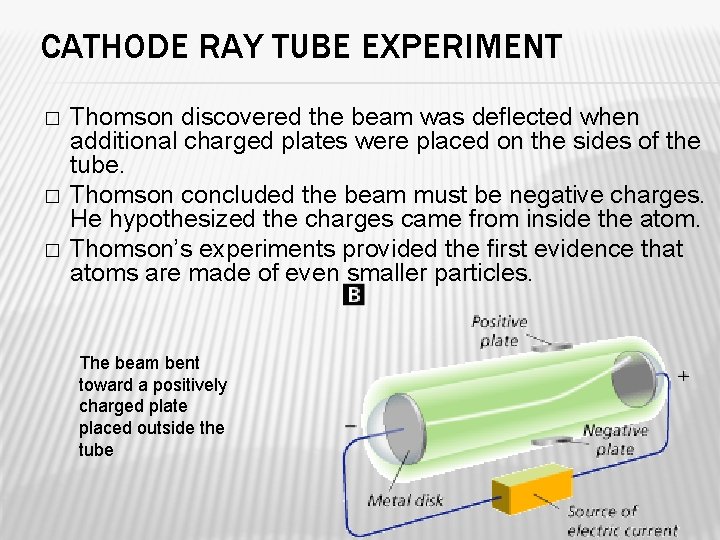
CATHODE RAY TUBE EXPERIMENT � � � Thomson discovered the beam was deflected when additional charged plates were placed on the sides of the tube. Thomson concluded the beam must be negative charges. He hypothesized the charges came from inside the atom. Thomson’s experiments provided the first evidence that atoms are made of even smaller particles. The beam bent toward a positively charged plate placed outside the tube

THOMSON’S MODEL His model of the atom looked like “plum pudding” (or chocolate chip ice cream). The pudding had an overall positive charge and the negative charges were randomly placed throughout. Overall, the atom is neutral.

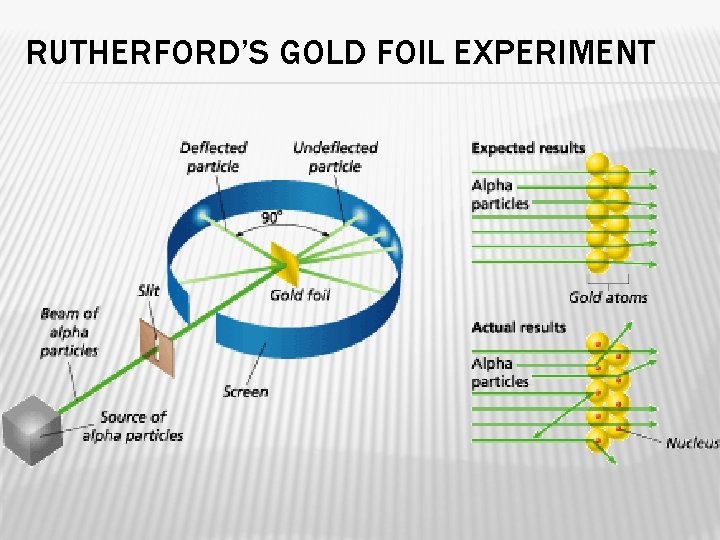
RUTHERFORD’S HYPOTHESIS Ø 1899 Ernest Rutherford discovered Uranium emits fast-moving particles that have a positive charge. (He called them alpha particles) � 1909 he and his students wanted to see what happens when the alpha particles are passed through a thin sheet of gold. He hypothesized most particles would travel in a straight path from their source. � Some would be deflected slightly. �

RUTHERFORD’S GOLD FOIL EXPERIMENT

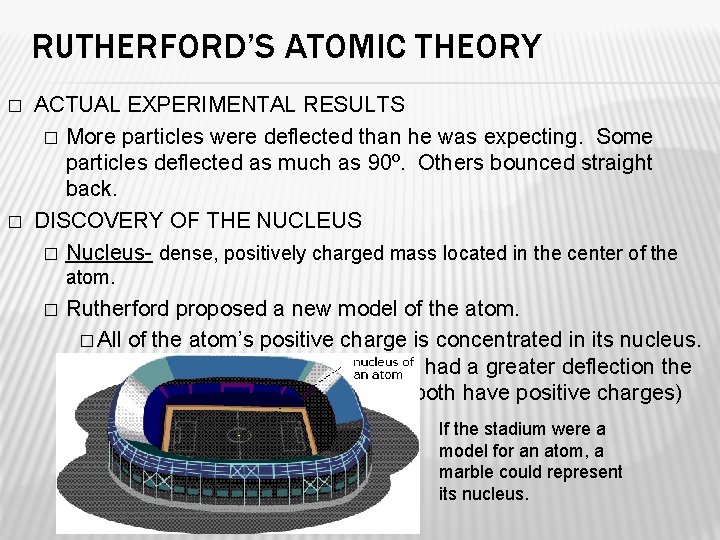
RUTHERFORD’S ATOMIC THEORY � � ACTUAL EXPERIMENTAL RESULTS � More particles were deflected than he was expecting. Some particles deflected as much as 90º. Others bounced straight back. DISCOVERY OF THE NUCLEUS � Nucleus- dense, positively charged mass located in the center of the atom. � Rutherford proposed a new model of the atom. � All of the atom’s positive charge is concentrated in its nucleus. This explains why alpha particles had a greater deflection the closer they were to the nucleus (both have positive charges) If the stadium were a model for an atom, a marble could represent its nucleus.

EXIT TICKET Answer these on the back of your notes packet: 1. Who discovered that subatomic particles exist? 2. List the atomic scientists in timeline order. 3. Contrast Democritus’ model to Rutherford’s model. 4. In your own words, describe the cathode ray tube experiment, and what it meant.

10/31 DO NOW The Cathode Ray Tube experiment: 1. 1) Who did this experiment? 2. 2) What was the result/discovery? The Gold Foil Experiment: 1. 3) Who did this experiment? 2. 4) What was the result/discovery? 5) Explain how Thompson’s “Chocolate Chip Ice cream” model is different from our current understanding of what an atom looks like

10/31 AGENDA 1. Do Now/ Hand in Do Nows 2. Sect 4. 2 – What does an atom look like? 3. Exit Ticket HW Packet pgs 4 -7 Quiz Tomorrow on Vocab! 4. 5.

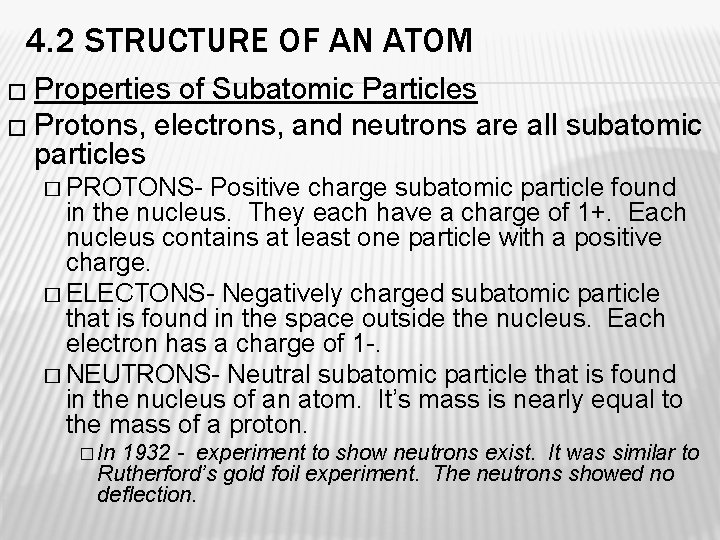
4. 2 STRUCTURE OF AN ATOM � Properties of Subatomic Particles � Protons, electrons, and neutrons are all subatomic particles � PROTONS- Positive charge subatomic particle found in the nucleus. They each have a charge of 1+. Each nucleus contains at least one particle with a positive charge. � ELECTONS- Negatively charged subatomic particle that is found in the space outside the nucleus. Each electron has a charge of 1 -. � NEUTRONS- Neutral subatomic particle that is found in the nucleus of an atom. It’s mass is nearly equal to the mass of a proton. � In 1932 - experiment to show neutrons exist. It was similar to Rutherford’s gold foil experiment. The neutrons showed no deflection.

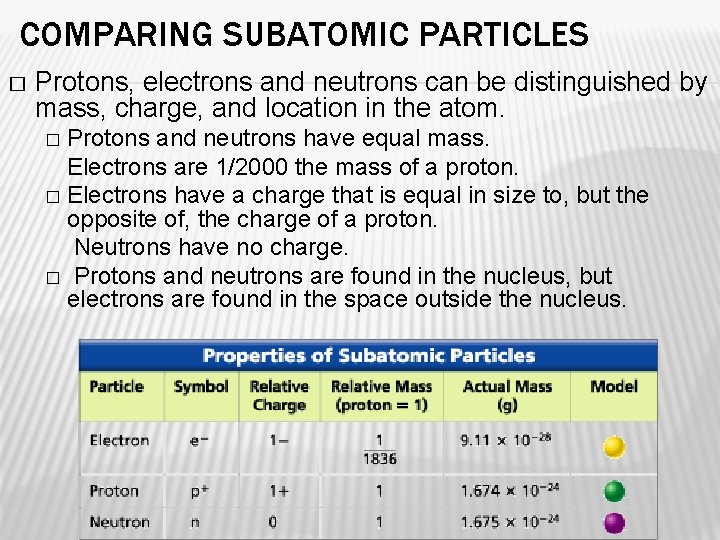
COMPARING SUBATOMIC PARTICLES � Protons, electrons and neutrons can be distinguished by mass, charge, and location in the atom. Protons and neutrons have equal mass. Electrons are 1/2000 the mass of a proton. � Electrons have a charge that is equal in size to, but the opposite of, the charge of a proton. Neutrons have no charge. � Protons and neutrons are found in the nucleus, but electrons are found in the space outside the nucleus. �

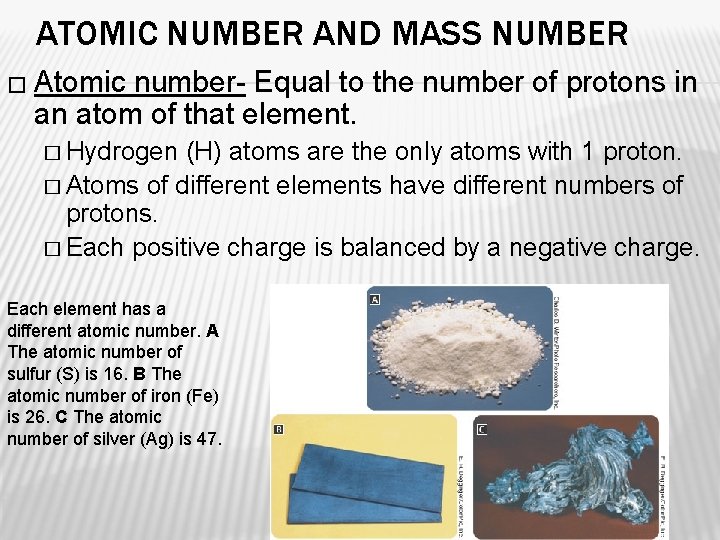
ATOMIC NUMBER AND MASS NUMBER � Atomic number- Equal to the number of protons in an atom of that element. � Hydrogen (H) atoms are the only atoms with 1 proton. � Atoms of different elements have different numbers of protons. � Each positive charge is balanced by a negative charge. Each element has a different atomic number. A The atomic number of sulfur (S) is 16. B The atomic number of iron (Fe) is 26. C The atomic number of silver (Ag) is 47.

� Mass number- Sum (add up) the protons and neutrons in the nucleus of that atom. �# of neutrons = mass # - atomic # � What’s with the decimals? * “Average Atomic Mass” � Isotopes � Every atom of a given element does have the same number of protons and electrons. � But every atom of a given element does not have the same number of neutrons. � Isotopes of an element have the same atomic number but a different mass number because they have different numbers of neutrons.

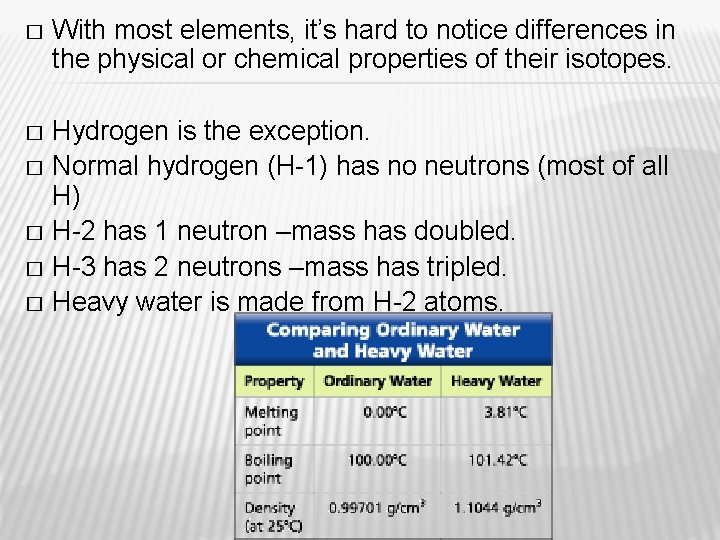
� With most elements, it’s hard to notice differences in the physical or chemical properties of their isotopes. Hydrogen is the exception. � Normal hydrogen (H-1) has no neutrons (most of all H) � H-2 has 1 neutron –mass has doubled. � H-3 has 2 neutrons –mass has tripled. � Heavy water is made from H-2 atoms. �

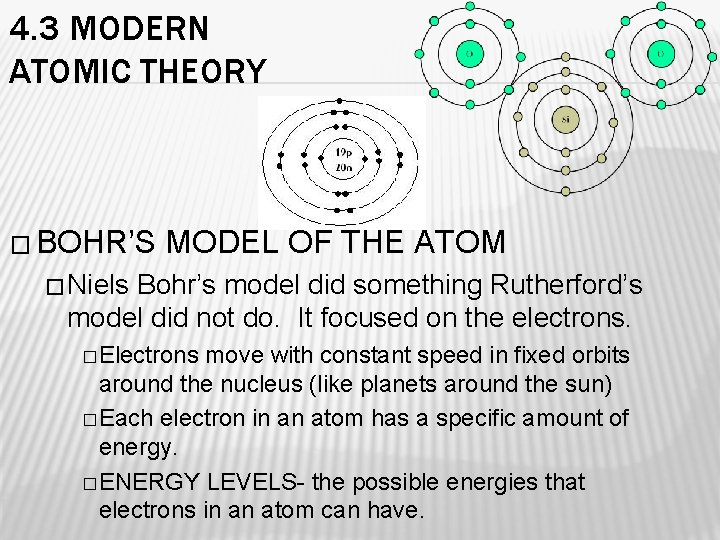
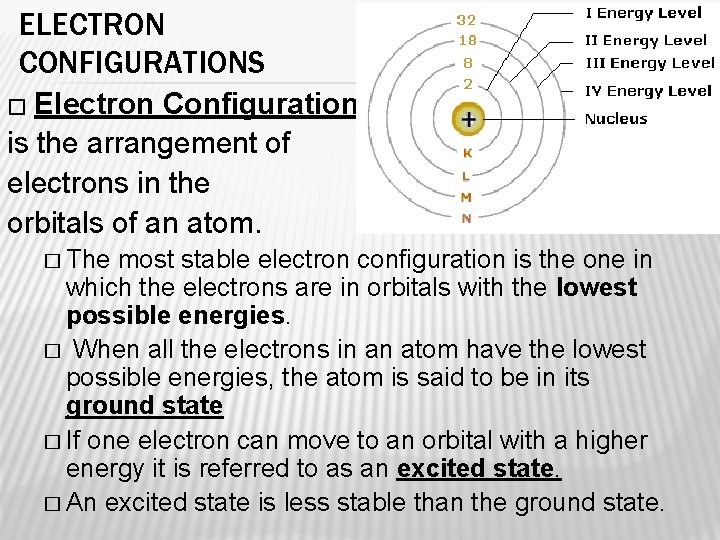
4. 3 MODERN ATOMIC THEORY � BOHR’S MODEL OF THE ATOM � Niels Bohr’s model did something Rutherford’s model did not do. It focused on the electrons. � Electrons move with constant speed in fixed orbits around the nucleus (like planets around the sun) � Each electron in an atom has a specific amount of energy. � ENERGY LEVELS- the possible energies that electrons in an atom can have.

UNDERSTANDING ENERGY LEVELS � Picture energy levels as steps in a staircase. � You can go up or down the steps, but only in wholestep increments. You cannot stand between steps on a staircase. Electrons cannot exist between energy levels. � An electron in an atom can move from one energy level to another when the atom gains or loses energy. � The size of the jump determines the amount of energy gained or lost. � Energy released as the electron jumps back down to its lower energy levels is often given off in the form of visible light. � Different elements emit different colors of light.

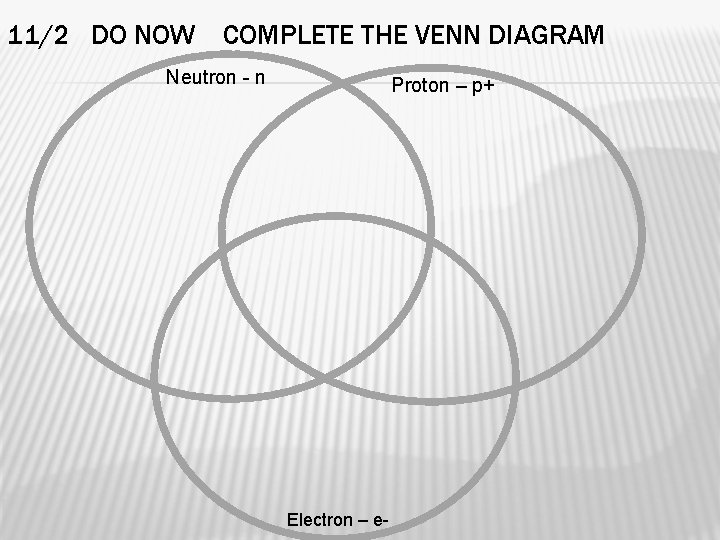
11/2 DO NOW COMPLETE THE VENN DIAGRAM Neutron - n Proton – p+ Electron – e-

11/2 AGENDA 1. 2. 3. 4. 5. Do Now Check and Go over HW Quiz Finish Chapter 4: Orbitals and the Electron Cloud Subatomic particles Practice (Chart Pg 8) *HW – Pgs 9, 10, 11, 15, 16, 17 *Another Quiz Tomorrow (Atomic Chart) *Test Friday

ELECTRON CLOUD MODEL � Bohr was incorrect in assuming electrons moved like planets in a solar system. They are actually less predictable. � ELETRON CLOUD- a visual model of the most likely locations for electrons in an atom. The cloud is denser at the locations where the probability of finding an electron is higher. � Scientists use the electron cloud model to describe the possible locations of electrons around the nucleus.

ELECTRON CLOUD ANALOGY When the propeller of an airplane is at rest, you can see the locations of the blades. When the propeller is moving, you see only a blur that is similar to a drawing of an electron cloud

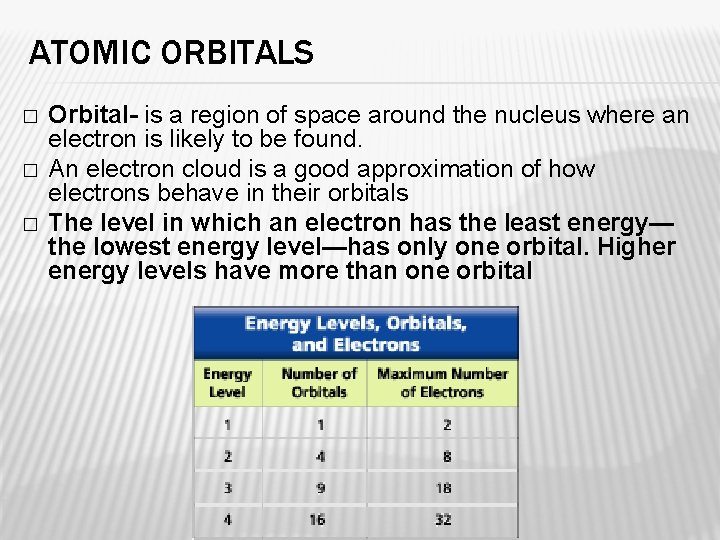
ATOMIC ORBITALS � � � Orbital- is a region of space around the nucleus where an electron is likely to be found. An electron cloud is a good approximation of how electrons behave in their orbitals The level in which an electron has the least energy— the lowest energy level—has only one orbital. Higher energy levels have more than one orbital

ELECTRON CONFIGURATIONS � Electron Configuration is the arrangement of electrons in the orbitals of an atom. � The most stable electron configuration is the one in which the electrons are in orbitals with the lowest possible energies. � When all the electrons in an atom have the lowest possible energies, the atom is said to be in its ground state � If one electron can move to an orbital with a higher energy it is referred to as an excited state. � An excited state is less stable than the ground state.

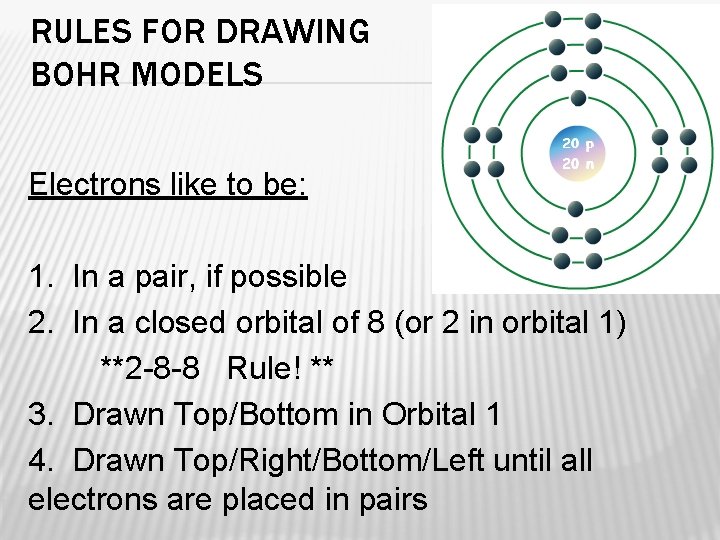
RULES FOR DRAWING BOHR MODELS Electrons like to be: 1. In a pair, if possible 2. In a closed orbital of 8 (or 2 in orbital 1) **2 -8 -8 Rule! ** 3. Drawn Top/Bottom in Orbital 1 4. Drawn Top/Right/Bottom/Left until all electrons are placed in pairs

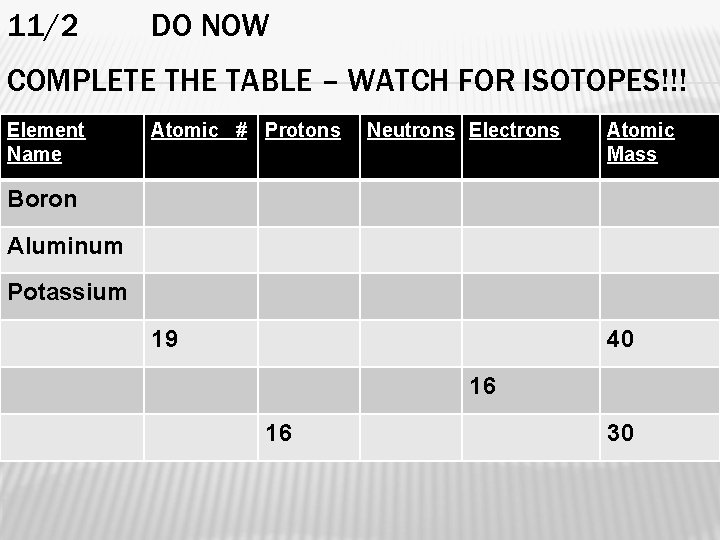
11/2 DO NOW COMPLETE THE TABLE – WATCH FOR ISOTOPES!!! Element Name Atomic # Protons Neutrons Electrons Atomic Mass Boron Aluminum Potassium 19 40 16 16 30

11/2 1. 2. 3. 4. AGENDA Do Now Check and Go over HW Quiz Drawing Bohr Diagrams - Worksheet pages 17, 18 *HW – Pgs 12, 13, 14, 19 *Test Friday

11/3 DO NOW DRAW THE BOHR DIAGRAMS Carbon Magnesium

11/3 1. 2. Do Now Correction to Bohr Diagrams a) 3. 4. AGENDA Orbital 1 (pg 18 and study guide) Go over quiz Kahoot! x 2 *HW –Study *Test Tomorrow

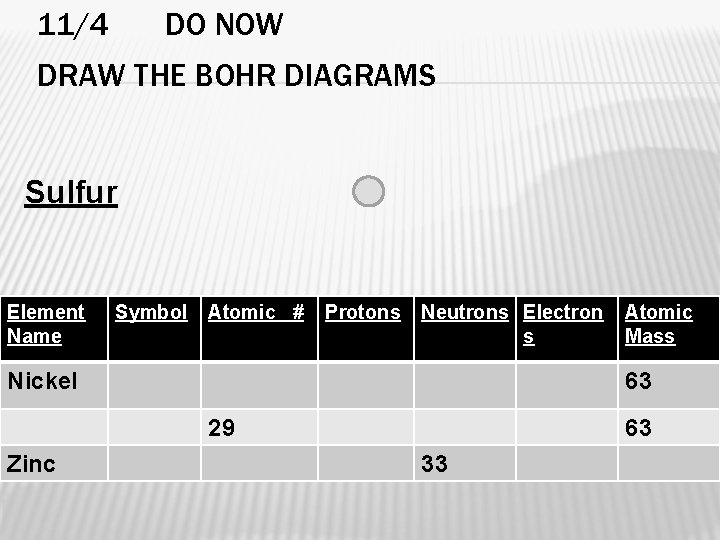
11/4 DO NOW DRAW THE BOHR DIAGRAMS Sulfur Element Name Symbol Atomic # Protons Neutrons Electron s Nickel 63 29 Zinc Atomic Mass 63 33

11/4 1. 2. 3. 4. AGENDA Do Now Hand in Do Now Test Flame color activity (if time)