100 years of nuclear isomers then and now

![Weart, 1983*: Von Weizäcker, in a theoretical textbook published [earlier] in 1936, declared that Weart, 1983*: Von Weizäcker, in a theoretical textbook published [earlier] in 1936, declared that](https://slidetodoc.com/presentation_image/3bc725a43812512772e3af66edb7e687/image-7.jpg)



- Slides: 32

100 years of nuclear isomers: then and now • early years • extreme cases • applications • recent observations Phil Walker University of Surrey, UK

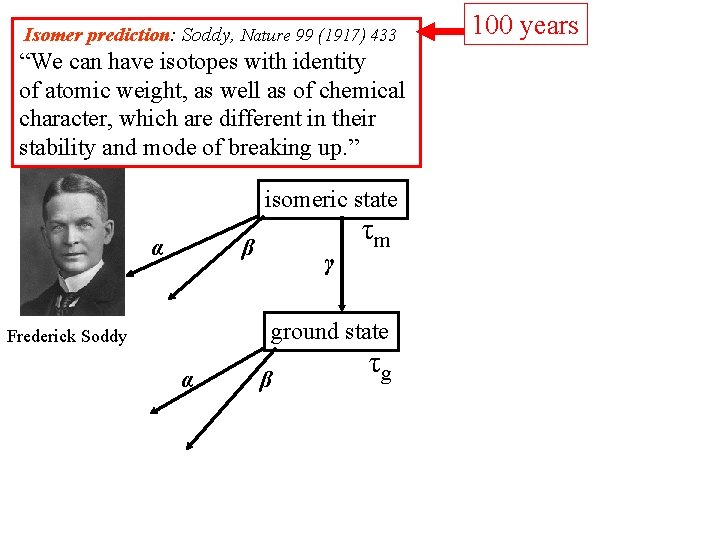
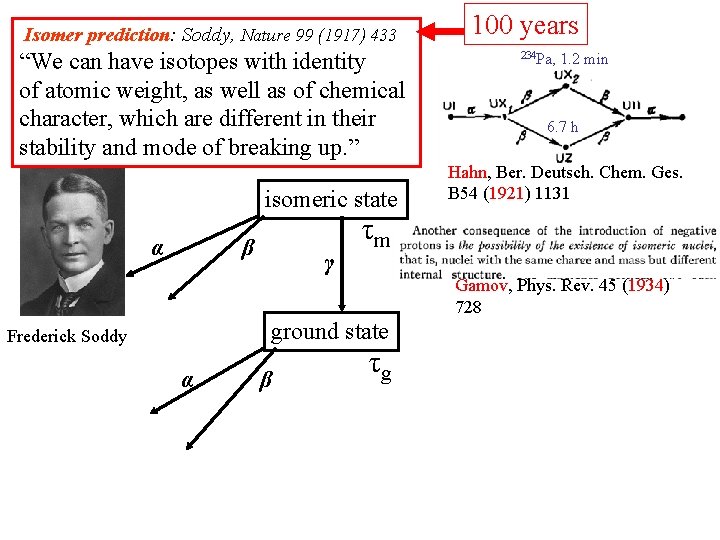
Isomer prediction: Soddy, Nature 99 (1917) 433 “We can have isotopes with identity of atomic weight, as well as of chemical character, which are different in their stability and mode of breaking up. ” isomeric state α β γ τm ground state Frederick Soddy α β τg 100 years

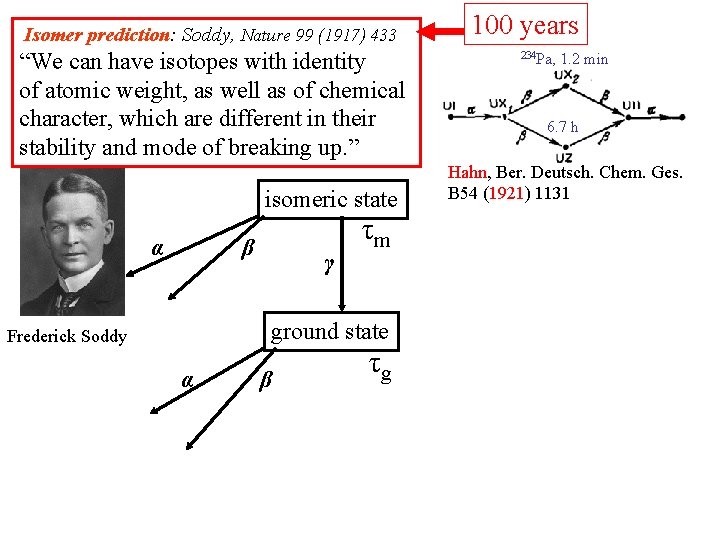
Isomer prediction: Soddy, Nature 99 (1917) 433 “We can have isotopes with identity of atomic weight, as well as of chemical character, which are different in their stability and mode of breaking up. ” isomeric state α β γ τm ground state Frederick Soddy α β τg 100 years 234 Pa, 1. 2 min 6. 7 h Hahn, Ber. Deutsch. Chem. Ges. B 54 (1921) 1131

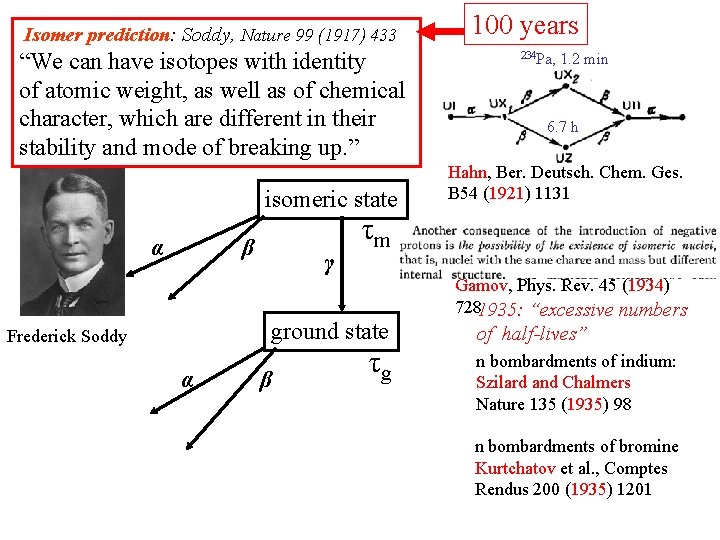
Isomer prediction: Soddy, Nature 99 (1917) 433 “We can have isotopes with identity of atomic weight, as well as of chemical character, which are different in their stability and mode of breaking up. ” isomeric state α β γ α β 234 Pa, 1. 2 min 6. 7 h Hahn, Ber. Deutsch. Chem. Ges. B 54 (1921) 1131 τm Gamov, Phys. Rev. 45 (1934) 728 ground state Frederick Soddy 100 years τg

Isomer prediction: Soddy, Nature 99 (1917) 433 “We can have isotopes with identity of atomic weight, as well as of chemical character, which are different in their stability and mode of breaking up. ” isomeric state α β γ α β 234 Pa, 1. 2 min 6. 7 h Hahn, Ber. Deutsch. Chem. Ges. B 54 (1921) 1131 τm ground state Frederick Soddy 100 years τg Gamov, Phys. Rev. 45 (1934) 7281935: “excessive numbers of half-lives” n bombardments of indium: Szilard and Chalmers Nature 135 (1935) 98 n bombardments of bromine Kurtchatov et al. , Comptes Rendus 200 (1935) 1201

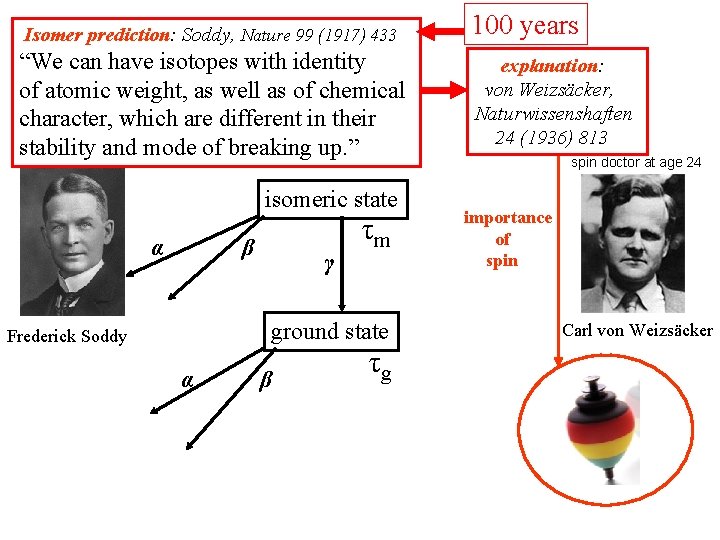
Isomer prediction: Soddy, Nature 99 (1917) 433 100 years “We can have isotopes with identity of atomic weight, as well as of chemical character, which are different in their stability and mode of breaking up. ” explanation: von Weizsäcker, Naturwissenshaften 24 (1936) 813 isomeric state α β γ τm ground state Frederick Soddy α β τg spin doctor at age 24 importance of spin Carl von Weizsäcker
![Weart 1983 Von Weizäcker in a theoretical textbook published earlier in 1936 declared that Weart, 1983*: Von Weizäcker, in a theoretical textbook published [earlier] in 1936, declared that](https://slidetodoc.com/presentation_image/3bc725a43812512772e3af66edb7e687/image-7.jpg)
Weart, 1983*: Von Weizäcker, in a theoretical textbook published [earlier] in 1936, declared that isomers “in fact do not seem to occur”. * S. R. Weart in “Discovery of nuclear fission”, contribution to “Otto Hahn and the Rise of Nuclear Physics”, W. R. Shea (Ed. ), D. Reidel Publishing Company, 1983 (University of Western Ontario Series in Philosophy of Science, Vol. 22) See also: M. Mladjenović, The Defining Years of Nuclear Physics, 1932 -1960’s (Io. P Publishing, Bristol 1998)

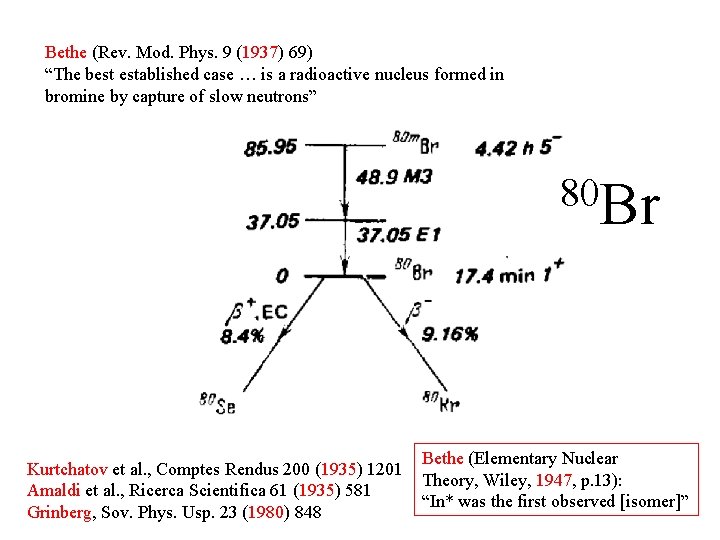
Bethe (Rev. Mod. Phys. 9 (1937) 69) “The best established case … is a radioactive nucleus formed in bromine by capture of slow neutrons” 80 Br Kurtchatov et al. , Comptes Rendus 200 (1935) 1201 Amaldi et al. , Ricerca Scientifica 61 (1935) 581 Grinberg, Sov. Phys. Usp. 23 (1980) 848 Bethe (Elementary Nuclear Theory, Wiley, 1947, p. 13): “In* was the first observed [isomer]”

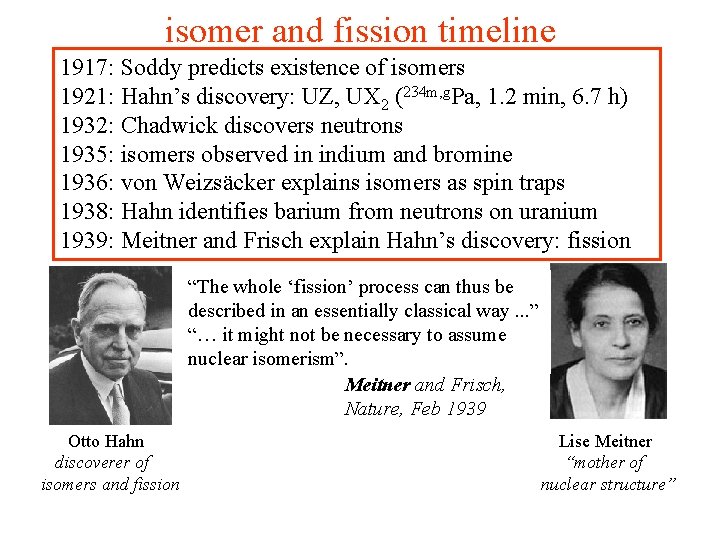
isomer and fission timeline 1917: Soddy predicts existence of isomers 1921: Hahn’s discovery: UZ, UX 2 (234 m, g. Pa, 1. 2 min, 6. 7 h) 1932: Chadwick discovers neutrons 1935: isomers observed in indium and bromine 1936: von Weizsäcker explains isomers as spin traps 1938: Hahn identifies barium from neutrons on uranium 1939: Meitner and Frisch explain Hahn’s discovery: fission “The whole ‘fission’ process can thus be described in an essentially classical way. . . ” “… it might not be necessary to assume nuclear isomerism”. Meitner and Frisch, Nature, Feb 1939 Otto Hahn discoverer of isomers and fission Lise Meitner “mother of nuclear structure”

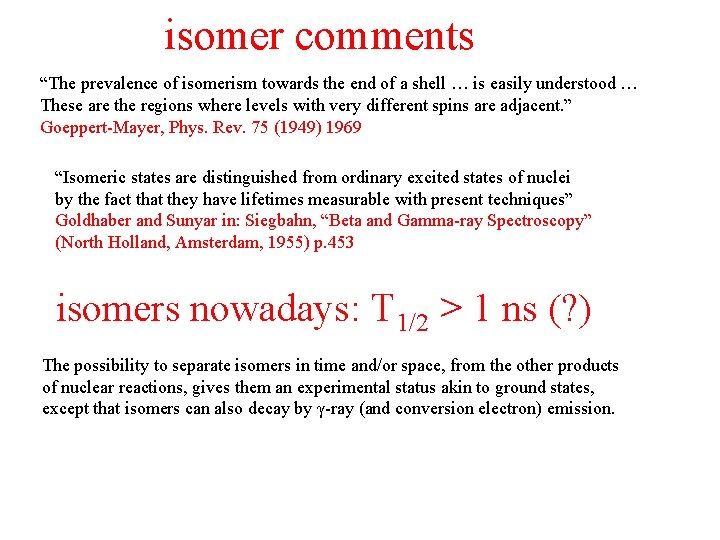
isomer comments “The prevalence of isomerism towards the end of a shell … is easily understood … These are the regions where levels with very different spins are adjacent. ” Goeppert-Mayer, Phys. Rev. 75 (1949) 1969 “Isomeric states are distinguished from ordinary excited states of nuclei by the fact that they have lifetimes measurable with present techniques” Goldhaber and Sunyar in: Siegbahn, “Beta and Gamma-ray Spectroscopy” (North Holland, Amsterdam, 1955) p. 453 isomers nowadays: T 1/2 > 1 ns (? ) The possibility to separate isomers in time and/or space, from the other products of nuclear reactions, gives them an experimental status akin to ground states, except that isomers can also decay by γ-ray (and conversion electron) emission.

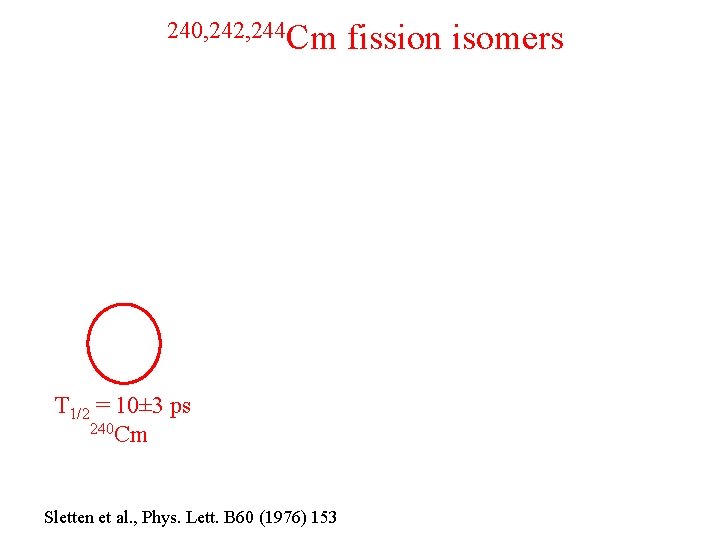
240, 242, 244 Cm T 1/2 = 10± 3 ps 240 Cm Sletten et al. , Phys. Lett. B 60 (1976) 153 fission isomers

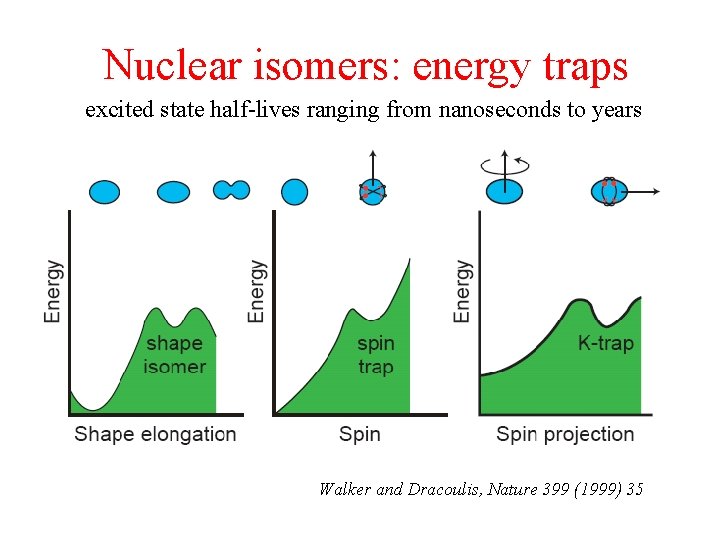
Nuclear isomers: energy traps excited state half-lives ranging from nanoseconds to years Walker and Dracoulis, Nature 399 (1999) 35

Shape isomers – e. g. fission isomers 242 Am, 14 ms (first, as well as longest lived) Polikanov et al. , JETP (Sov. Phys. ) 42 (1962) 164 Spin isomers: first examples: 234 Pa, 115 In, 80 Br longest lived: 180 Ta, 9 -, > 4. 5 x 1016 y Lehnert et al. Phys. Rev. C 95 (2017) 044306 K isomers: 180 Hf, 5. 5 h [190 Os, 10 min] first examples Burson et al. , Phys. Rev. 83 (1951) 62 [Chu, Phys. Rev. 79 (1950) 582] theory: Alaga et al. , Mat. Fys. Medd. Dan. Vid. Selsk. 29 (1955) No. 9* longest lived: 178 Hf, 16+, 31 y, Helmer and Reich, Nucl. Phys. A 211 (1973) 1 * degree of K-forbiddenness, ν = ΔK - L

> 10 ns energy Me. V Jain et al. , Nucl. Data Sheets 128 (2015) 1

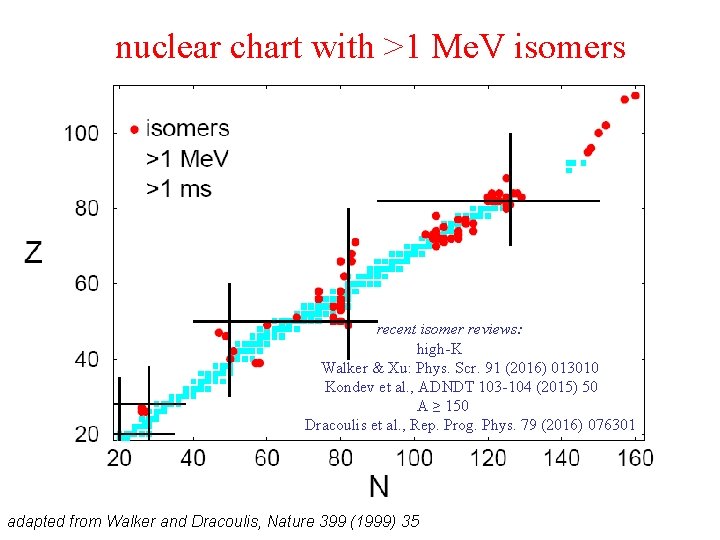
nuclear chart with >1 Me. V isomers recent isomer reviews: high-K Walker & Xu: Phys. Scr. 91 (2016) 013010 Kondev et al. , ADNDT 103 -104 (2015) 50 A ≥ 150 Dracoulis et al. , Rep. Prog. Phys. 79 (2016) 076301 adapted from Walker and Dracoulis, Nature 399 (1999) 35

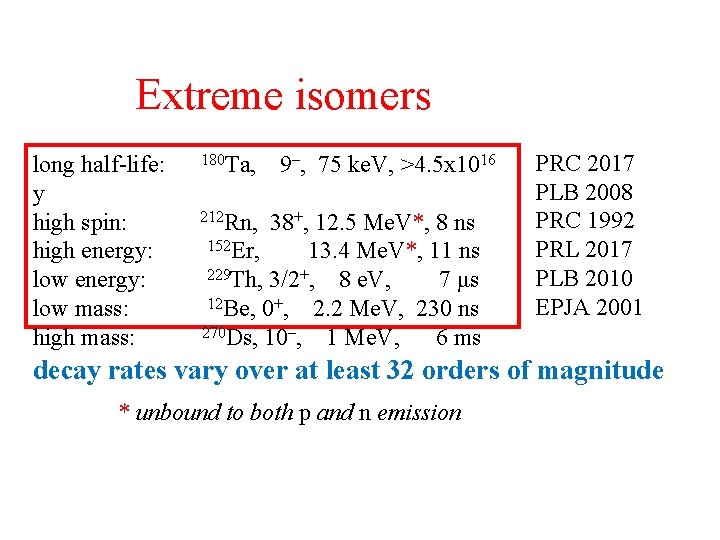
Extreme isomers long half-life: y high spin: high energy: low mass: high mass: 180 Ta, 9–, 75 ke. V, >4. 5 x 1016 212 Rn, 38+, 12. 5 Me. V*, 8 ns 152 Er, 13. 4 Me. V*, 11 ns 229 Th, 3/2+, 8 e. V, 7 μs 12 Be, 0+, 2. 2 Me. V, 230 ns 270 Ds, 10–, 1 Me. V, 6 ms PRC 2017 PLB 2008 PRC 1992 PRL 2017 PLB 2010 EPJA 2001 decay rates vary over at least 32 orders of magnitude * unbound to both p and n emission

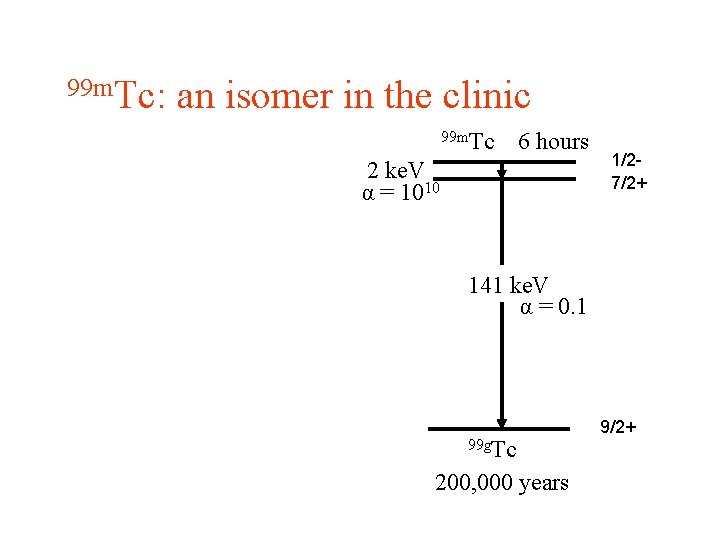
99 m. Tc: an isomer in the clinic 99 m. Tc 6 hours 2 ke. V α = 1010 1/27/2+ 141 ke. V α = 0. 1 99 g. Tc 200, 000 years 9/2+

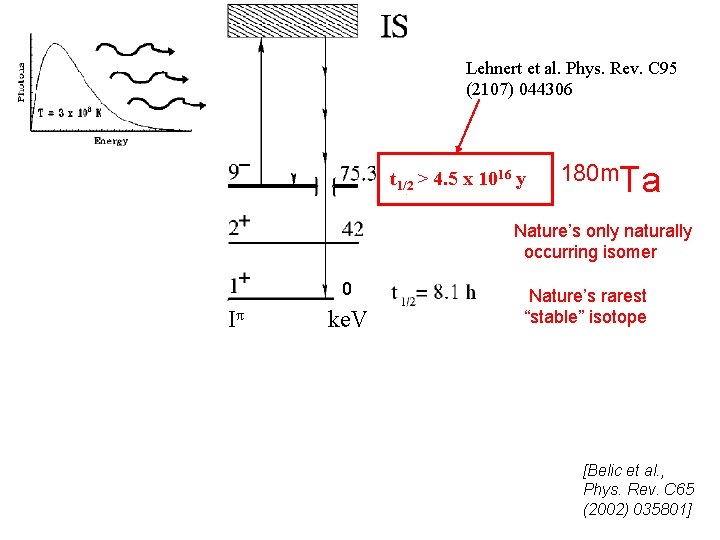
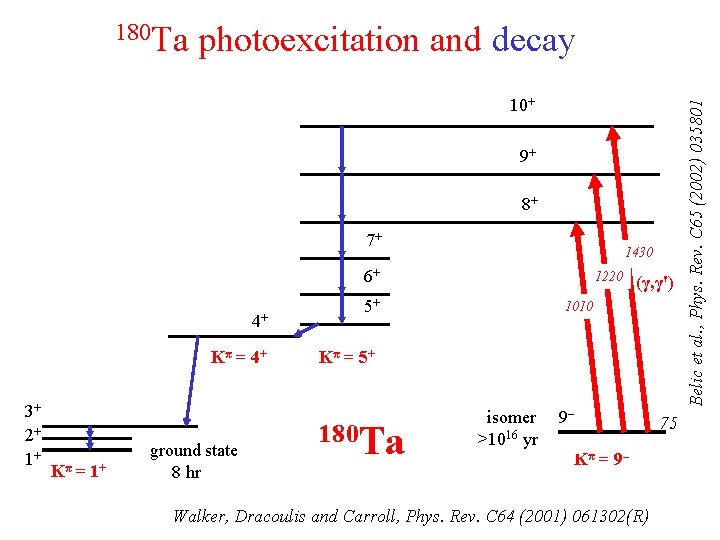
Lehnert et al. Phys. Rev. C 95 (2107) 044306 t 1/2 > 4. 5 x 1016 y 180 m. Ta Nature’s only naturally occurring isomer 0 Iπ ke. V Nature’s rarest “stable” isotope J [Belic et al. , Phys. Rev. C 65 (2002) 035801]

photoexcitation and decay 10+ 9+ 8+ 7+ 1430 6+ 4+ Kπ = 4+ 3+ 2+ 1+ Kπ = 1+ ground state 8 hr 1220 5+ (γ, γ') 1010 Kπ = 5+ 180 Ta isomer >1016 yr 9– 75 Kπ = 9– Walker, Dracoulis and Carroll, Phys. Rev. C 64 (2001) 061302(R) Belic et al. , Phys. Rev. C 65 (2002) 035801 180 Ta

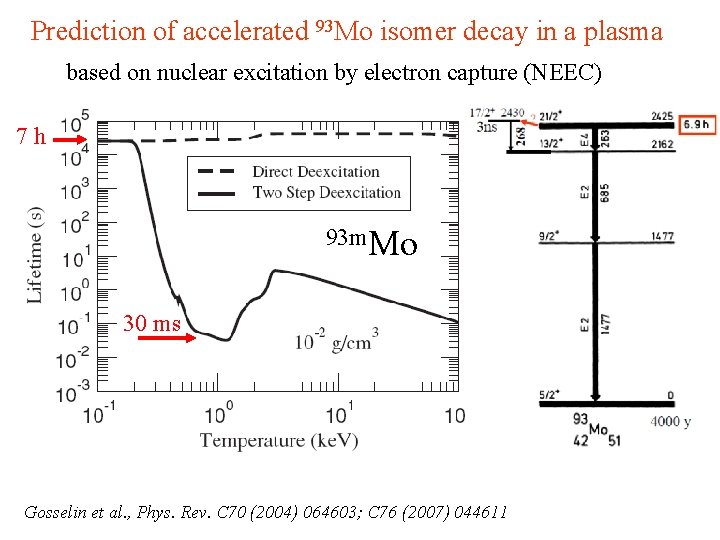
Prediction of accelerated 93 Mo isomer decay in a plasma based on nuclear excitation by electron capture (NEEC) 7 h 93 Mo in a plasma 93 m. Mo 30 ms Gosselin et al. , Phys. Rev. C 70 (2004) 064603; C 76 (2007) 044611

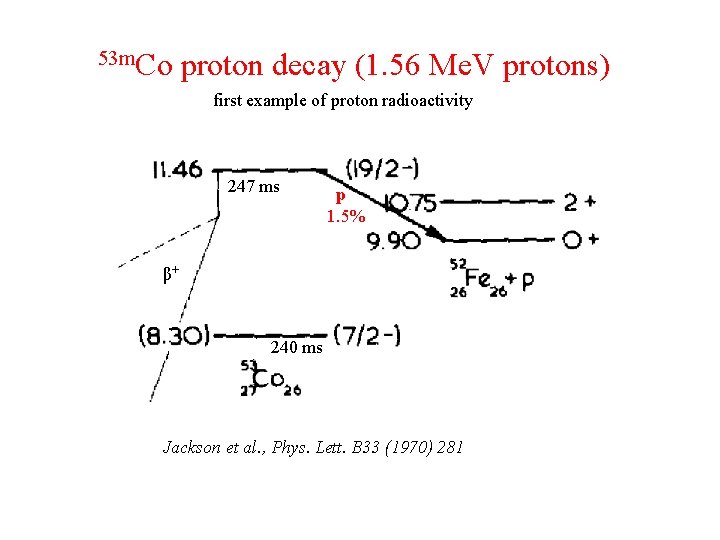
53 m. Co proton decay (1. 56 Me. V protons) first example of proton radioactivity 247 ms p 1. 5% β+ 240 ms Jackson et al. , Phys. Lett. B 33 (1970) 281

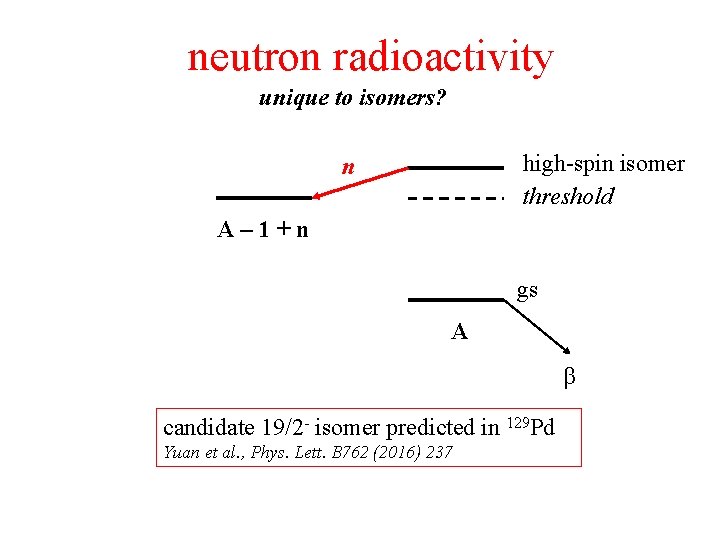
neutron radioactivity unique to isomers? high-spin isomer threshold n A– 1+n gs A β candidate 19/2 - isomer predicted in 129 Pd Yuan et al. , Phys. Lett. B 762 (2016) 237

recent isomer data a variety of examples

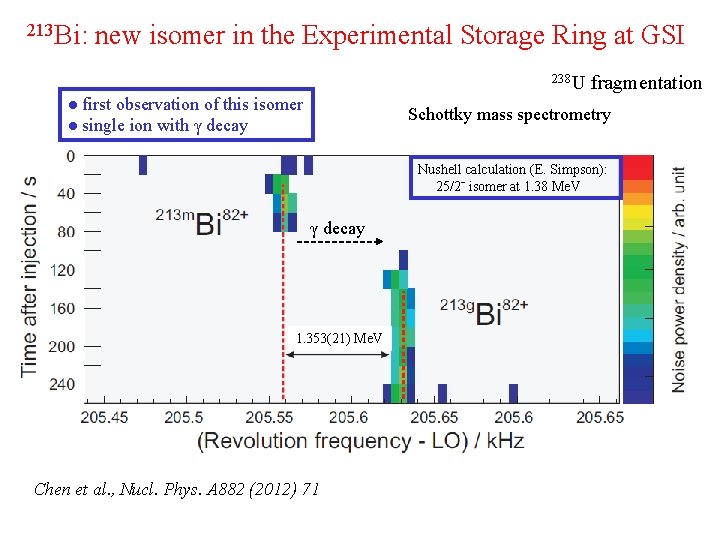
213 Bi: new isomer in the Experimental Storage Ring at GSI 238 U ● first observation of this isomer ● single ion with γ decay fragmentation Schottky mass spectrometry Nushell calculation (E. Simpson): 25/2ˉ isomer at 1. 38 Me. V γ decay 1. 353(21) Me. V Chen et al. , Nucl. Phys. A 882 (2012) 71

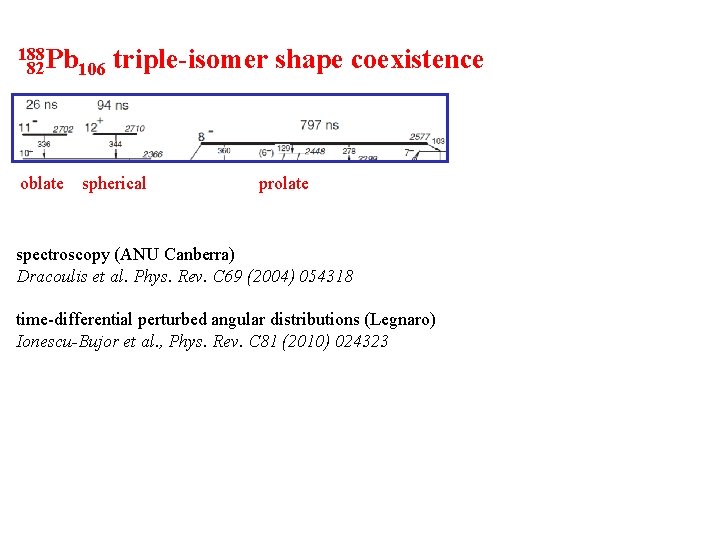
188 Pb 82 106 oblate triple-isomer shape coexistence spherical prolate spectroscopy (ANU Canberra) Dracoulis et al. Phys. Rev. C 69 (2004) 054318 time-differential perturbed angular distributions (Legnaro) Ionescu-Bujor et al. , Phys. Rev. C 81 (2010) 024323

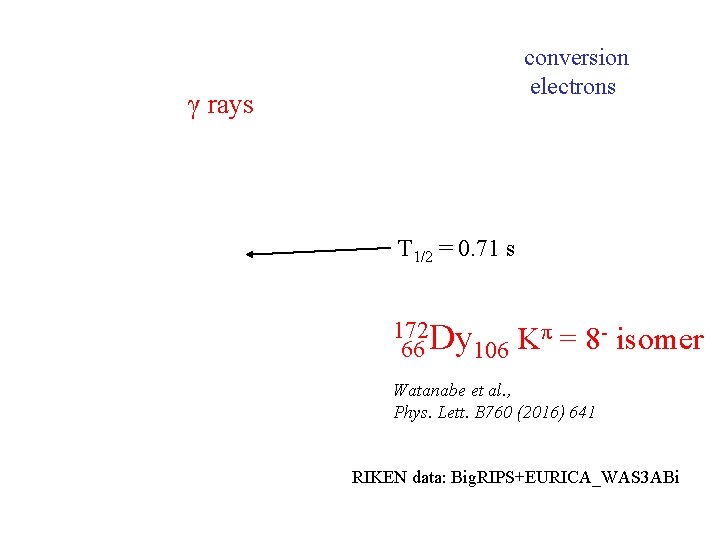
conversion electrons γ rays T 1/2 = 0. 71 s 172 Dy 66 106 Kπ = 8 - isomer Watanabe et al. , Phys. Lett. B 760 (2016) 641 RIKEN data: Big. RIPS+EURICA_WAS 3 ABi

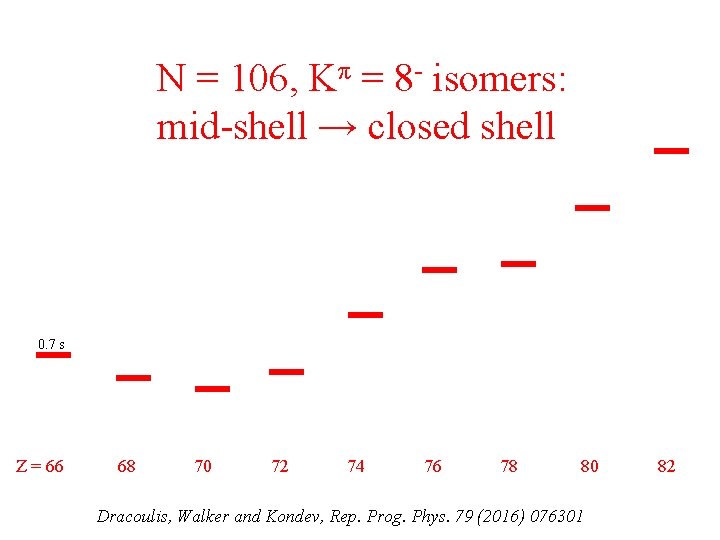
N = 106, Kπ = 8 - isomers: mid-shell → closed shell 0. 7 s Z = 66 68 70 72 74 76 78 80 Dracoulis, Walker and Kondev, Rep. Prog. Phys. 79 (2016) 076301 82

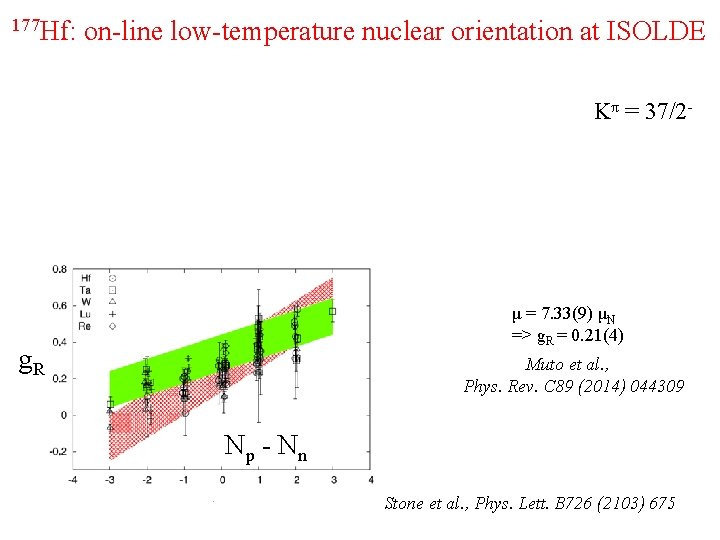
177 Hf: on-line low-temperature nuclear orientation at ISOLDE Kπ = 37/2 - μ = 7. 33(9) μN => g. R = 0. 21(4) g. R Muto et al. , Phys. Rev. C 89 (2014) 044309 Np - Nn Stone et al. , Phys. Lett. B 726 (2103) 675

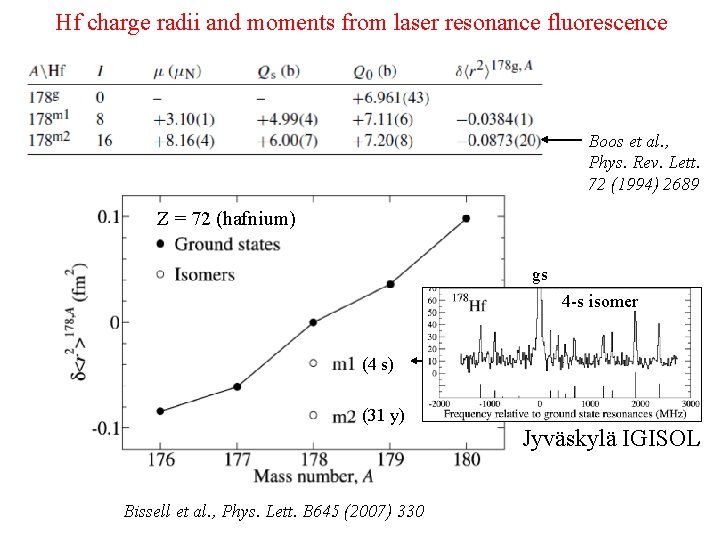
Hf charge radii and moments from laser resonance fluorescence Boos et al. , Phys. Rev. Lett. 72 (1994) 2689 Z = 72 (hafnium) gs 4 -s isomer (4 s) (31 y) Bissell et al. , Phys. Lett. B 645 (2007) 330 Jyväskylä IGISOL

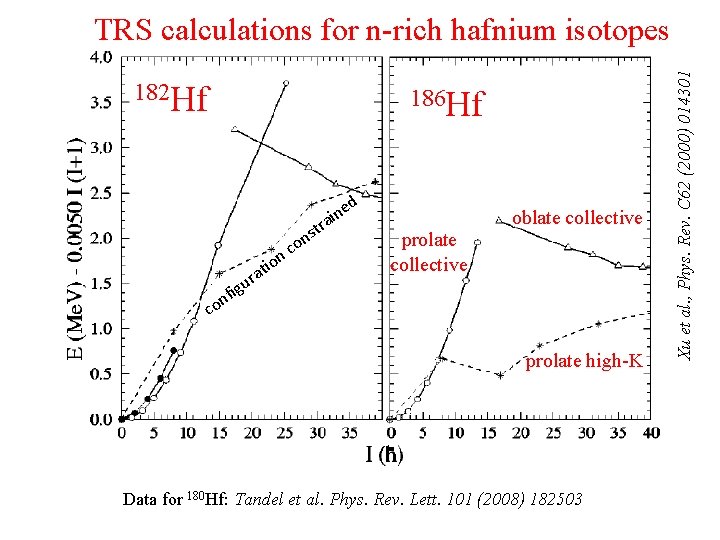
182 Hf n-rich Hf: E 186 vs Hf I io o nc at r u fig t ns ed n i ra prolate collective oblate collective n co prolate high-K Data for 180 Hf: Tandel et al. Phys. Rev. Lett. 101 (2008) 182503 Xu et al. , Phys. Rev. C 62 (2000) 014301 TRS calculations for n-rich hafnium isotopes

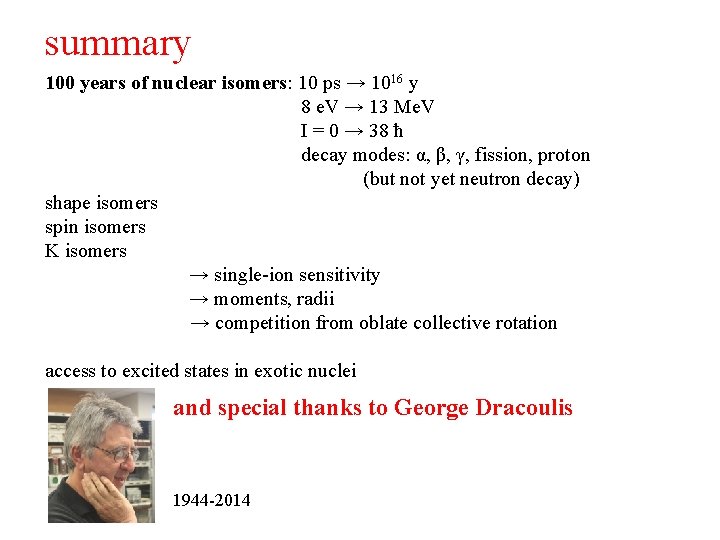
summary 100 years of nuclear isomers: 10 ps → 1016 y 8 e. V → 13 Me. V I = 0 → 38 ħ decay modes: α, β, γ, fission, proton (but not yet neutron decay) shape isomers spin isomers K isomers → single-ion sensitivity → moments, radii → competition from oblate collective rotation access to excited states in exotic nuclei

summary 100 years of nuclear isomers: 10 ps → 1016 y 8 e. V → 13 Me. V I = 0 → 38 ħ decay modes: α, β, γ, fission, proton (but not yet neutron decay) shape isomers spin isomers K isomers → single-ion sensitivity → moments, radii → competition from oblate collective rotation access to excited states in exotic nuclei and special thanks to George Dracoulis 1944 -2014
 100 100 100 100 100
100 100 100 100 100 Constitutional isomers vs conformational isomers
Constitutional isomers vs conformational isomers Héroïne dans la guerre de 100 ans (100 years war).
Héroïne dans la guerre de 100 ans (100 years war). Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Where is this image
Where is this image Sheep years to human years
Sheep years to human years 300 solar years to lunar years
300 solar years to lunar years Sociology: then and now
Sociology: then and now What is sociology section 2
What is sociology section 2 The seaside then and now
The seaside then and now Seaside now and then
Seaside now and then John purdy inventor
John purdy inventor Dubai then and now
Dubai then and now Agriculture then and now
Agriculture then and now Child labor drawings
Child labor drawings The seaside then and now
The seaside then and now Who invented pe
Who invented pe Then and now grammar
Then and now grammar Ffa regions
Ffa regions Jerusalem then and now
Jerusalem then and now Victorian seaside holidays
Victorian seaside holidays Now and then board
Now and then board Greece then and now
Greece then and now Now i see it now you don't
Now i see it now you don't That was then this is now summary
That was then this is now summary The story of an hour summary
The story of an hour summary Reported speech today
Reported speech today Then now
Then now Secondary education system in india
Secondary education system in india Colonel aureliano buendia
Colonel aureliano buendia Family life 100 years ago
Family life 100 years ago Consequences of the 100 years war
Consequences of the 100 years war