100 200 200 100 200 300 300 400













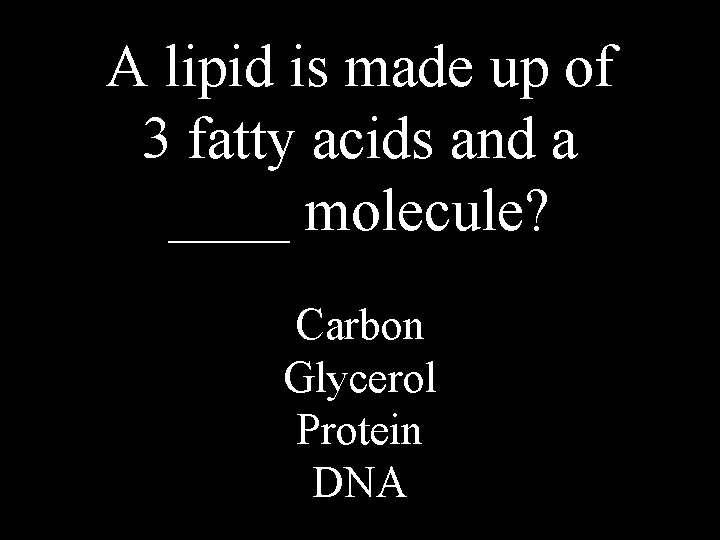















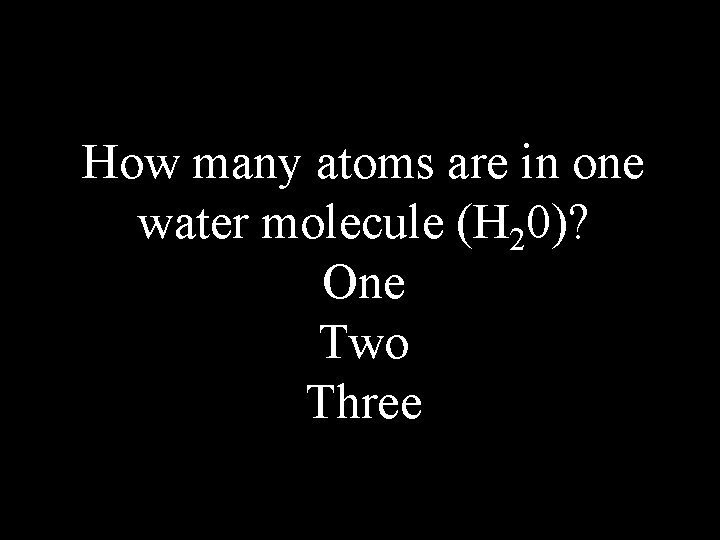

















































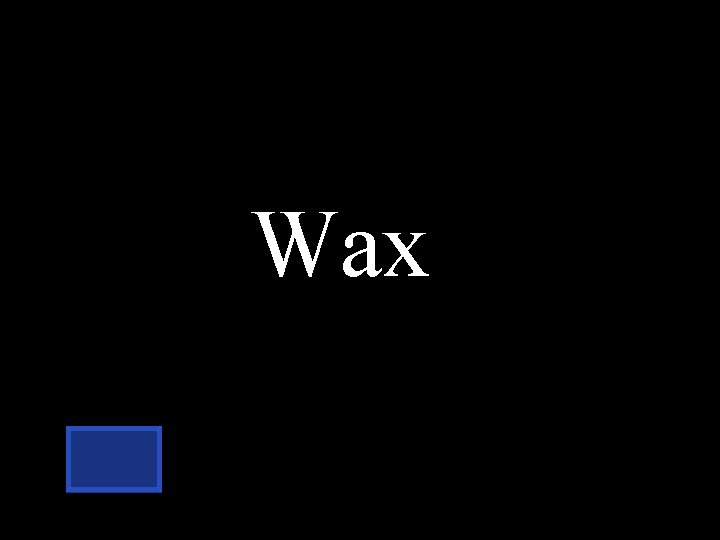
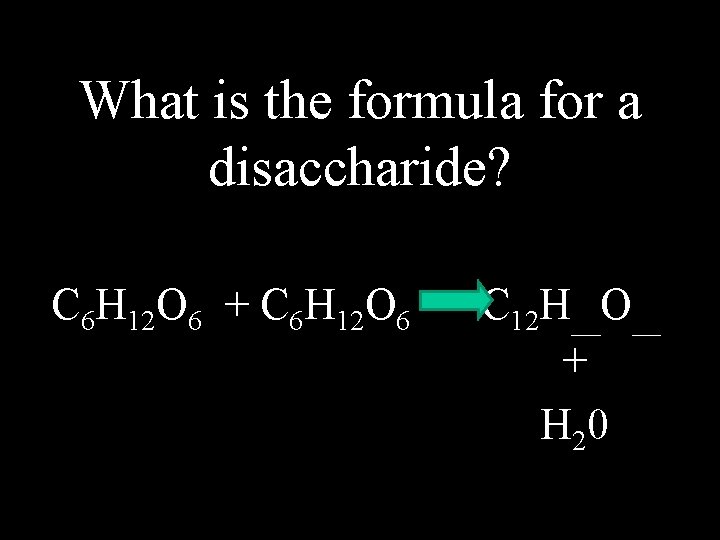
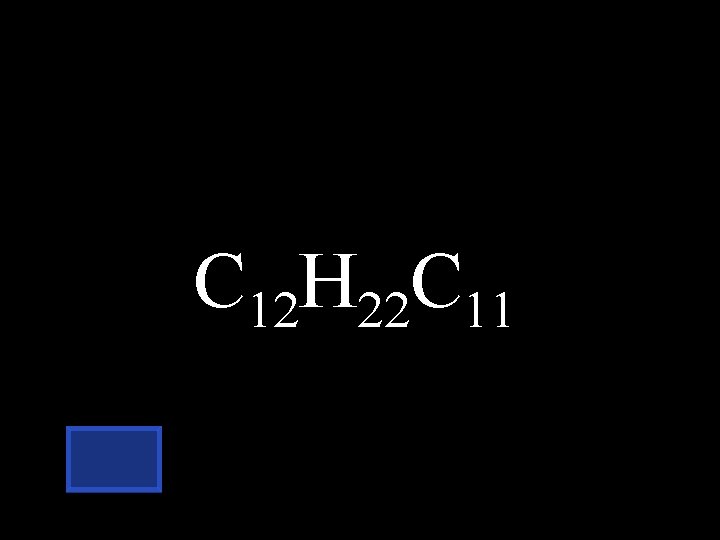
- Slides: 95







$100 $200 $200 $100 $200 $300 $300 $400 $400 $500 $500

When an atom has 12 protons and 13 electrons? positive negative neutral

Negative

When ions are formed, it usually leads to _____ bonding. Covalent Ionic Hydrogen Carbon

Ionic bond

What is the polarity of water good for?

1. Excellent solvent 2. Capillary action (helps water move up tree) 3. Cleans your face 4. Water tension 5. Water temperature

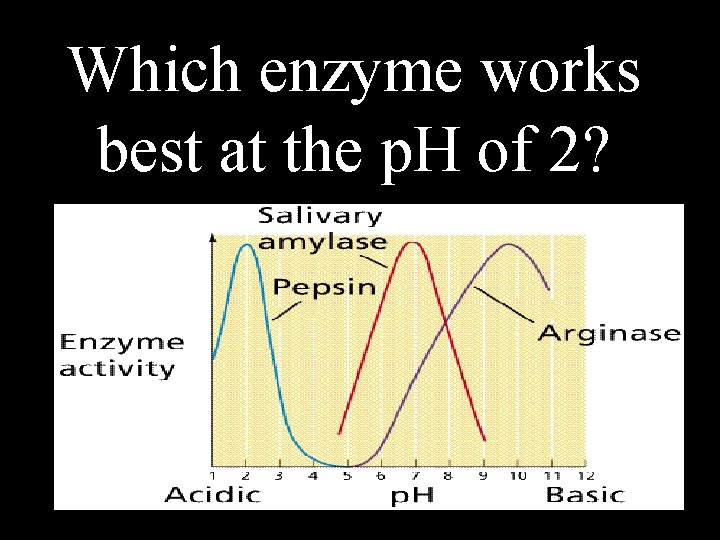
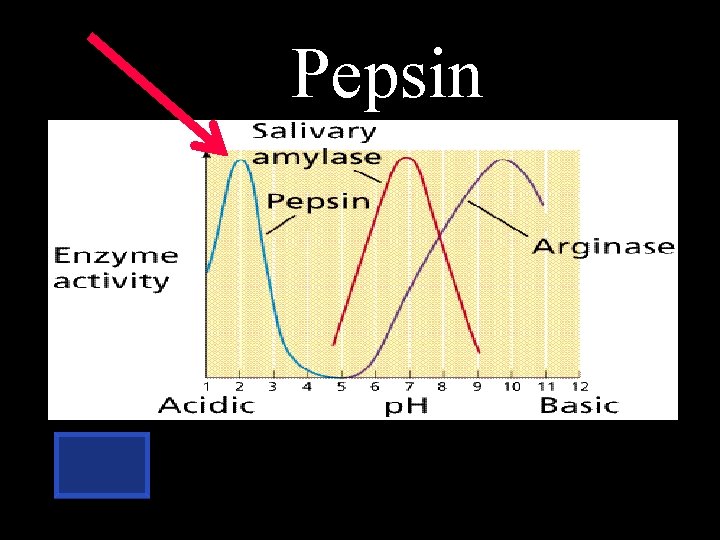
Which enzyme works best at the p. H of 2?

Pepsin

T or F The process of hydrolysis breaks apart polysaccharides. Think about the word hydro lysis
True
A lipid is made up of 3 fatty acids and a ____ molecule? Carbon Glycerol Protein DNA

glycerol

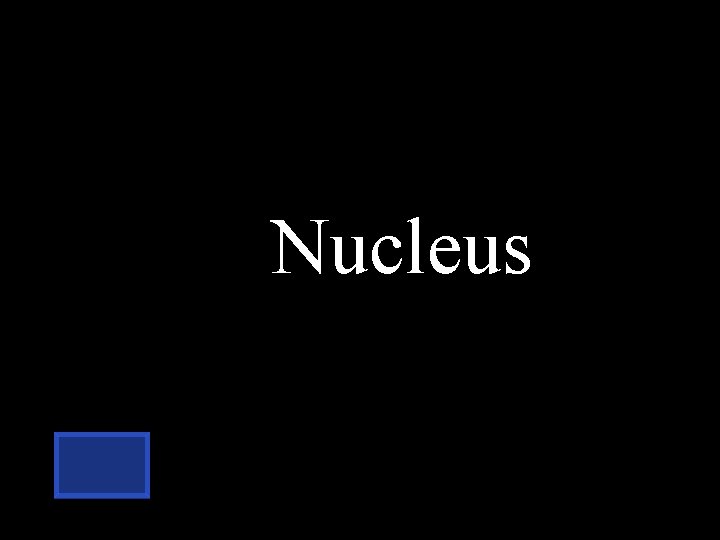
What part of the cell do we find nucleic acids? Plasma membrane Cytoplasm Nucleus

Nucleus

Which state of matter contain particles that hardly move and are tightly packed? Solid Liquid Gas

Solid

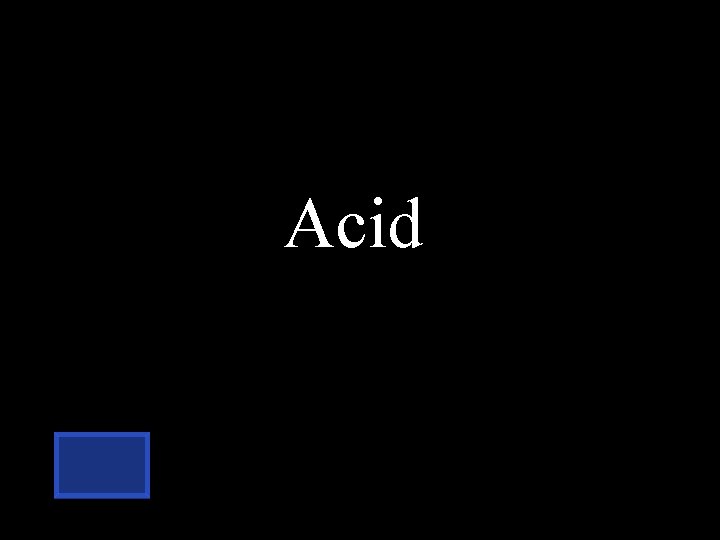
If there are too many hydrogen ions(H+) in solution, it is considered to be… Acid Alkaline neutral

Acid

Which is not an example of a chemical reaction? Burning wood Cutting paper into smaller pieces Rusting of metal Baking bread

Cutting paper into smaller pieces

If an atom has gained or lost an electron, it is considered to be. . . Neutral Positive Ion negative

Ion

What charge does an atom contain when it has gained an electron? Neutral Positive negative

Negative Charge

What is the process that combines 2 monosaccharides to form a disaccharide? Hydrolysis Condensation Carbonation Cell respiration

condensation

Which function is not an important function of lipids in a living organism? Insulation Quick energy Stores energy Found in membranes

Quick energy
How many atoms are in one water molecule (H 20)? One Two Three

Three

Explain why glucose and fructose are isomers of one another?

Same molecular formula different 3 -d arrangement

The basic unit of Nucleic acids are. . . Proteins Nucleotides Lipids Carbohydrates

Nucleotides

Proteins contain C, H, O and what other element. Copper Lead Nitrogen Chlorine

Nitrogen

When sodium has 11 protons & 10 electrons, what is the overall charge. positive negative neutral
It becomes (+) Positive

Name the 3 parts of an atom.

Protons Neutrons Electrons

When chlorine has 17 protons & 18 electrons, what is the overall charge. positive negative neutral
It becomes (-) Negative

Give me an example of an element An apple Rust Air Hydrogen

Hydrogen

What are polysaccharides? Lipids Carbohydrates Proteins Nucleic acids

Carbohydrates or sugars

Inorganic compounds usually DO NOT contain this element. Copper Carbon Nitrogen Chlorine

Carbon

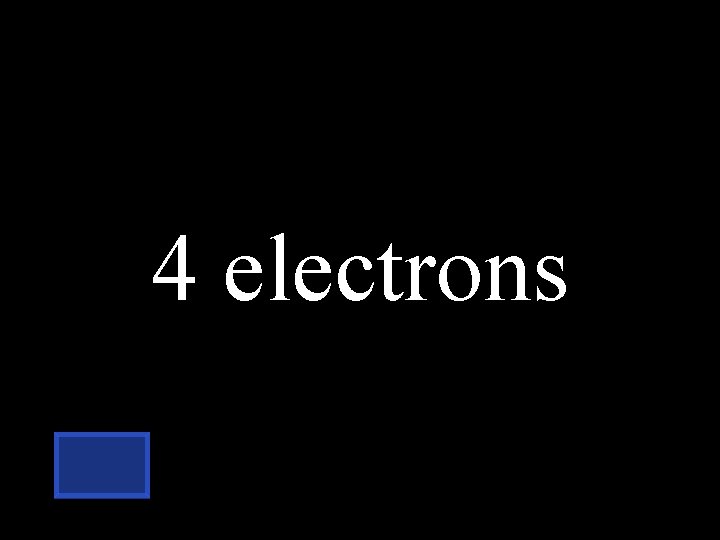
If the atomic # is 6, how many electrons does carbon have in its outer energy level? TWO THREE FOUR SIX

4 electrons

A p. H of 11 is considered …. Alkaline(basic) Acid neutral

Alkaline(basic)

Which one is not part of a nucleotide? Phosphate Nitrogen base Sugar(Ribose) Lipid

Lipid

An acid has a ph of __. Above 7 Below 7 At 7 Exactly 14

Below seven

Why is water considered polar? It has 2 personalities One side is (-), One side is (+) Even distribution of charges It has a south pole

It has a negative and positive side

When smaller molecules bond together to form a large molecule this is called? Hydrolysis Cell respiration Condensation

condensation






$200 $1000 $ $400 $ $ $600 $ $ $800 $ $ $1000 $ $ $400

A chemical reaction in which polypeptides are broken down into amino acids with the addition of water? Hydrolysis Hydronation Condensation

Hydrolysis

What is one example of a disaccharide? Glucose Sucrose Polysaccharide

Sucrose

______ is a steroids (a type of lipid) that is found in cell membranes & can also clog arteries. Carbohydrates Cholesterol Proteins Nucleic acids

Cholesterol

An area on an enzyme where the substrate fits. Enzyme loci Substrate loci Active site Enzyme-substrate loci

Active site

T or F All organic compounds contain the element Carbon.

True

What other factor; besides p. H, limits enzyme function?

Temperature

Sucrose + H 2 O Glucose + Fructose The reaction above is called … Hydrolysis Hydronation Condensation

Hydrolysis Reaction

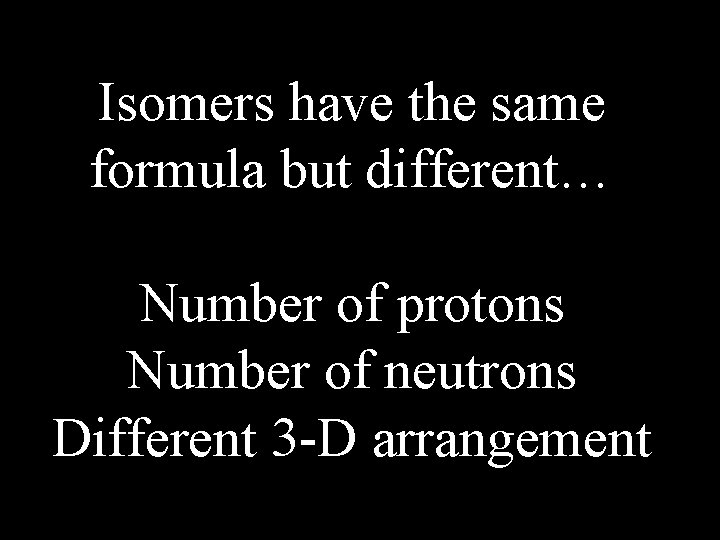
Isomers have the same formula but different… Number of protons Number of neutrons Different 3 -D arrangement

3 -D arrangement

If a solution has a lot of Hydroxide ions(OH ), it is considered to be… Acid Alkaline Neutral

Alkaline or Base

What is the type of lipid that comes from bees?
Wax
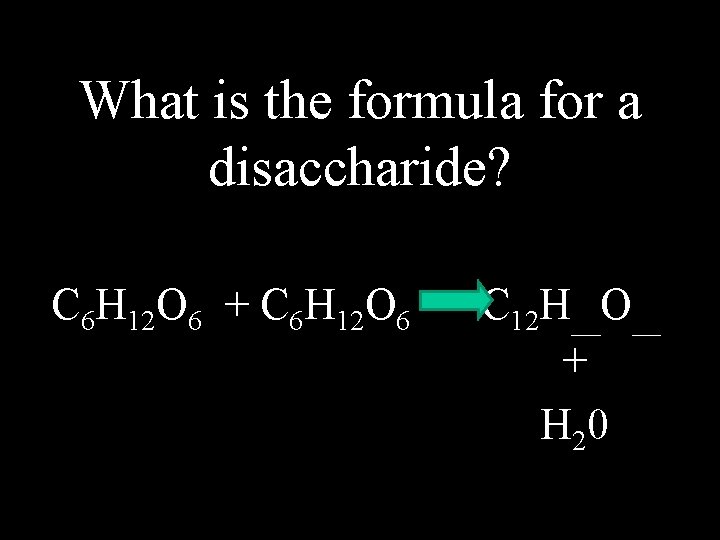
What is the formula for a disaccharide? C 6 H 12 O 6 + C 6 H 12 O 6 C 12 H__O__ + H 20
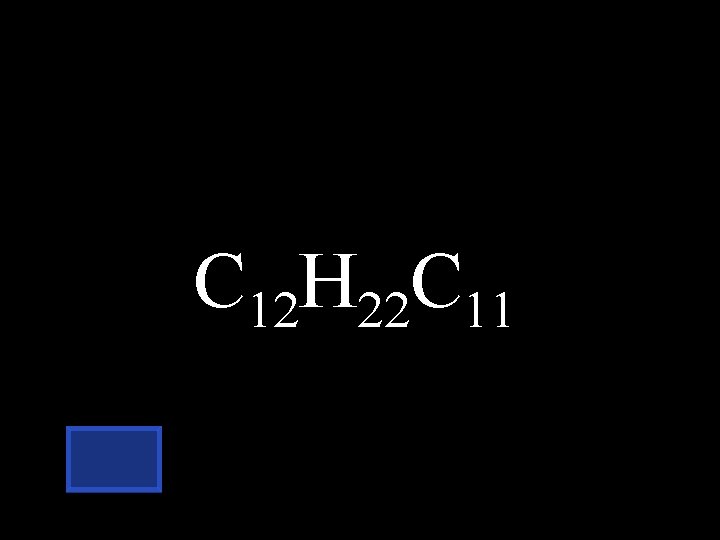
C 12 H 22 C 11