100 100 200 200 300 300 400 400































![What does the following symbol stand for? [H 3 + O ] What does the following symbol stand for? [H 3 + O ]](https://slidetodoc.com/presentation_image_h/fa6110a26dbbdc546c692933117f28ba/image-34.jpg)
![[H 3 + O ] Hydronium Ion Concentration [H 3 + O ] Hydronium Ion Concentration](https://slidetodoc.com/presentation_image_h/fa6110a26dbbdc546c692933117f28ba/image-35.jpg)
![What does the following symbol stand for? [OH ] What does the following symbol stand for? [OH ]](https://slidetodoc.com/presentation_image_h/fa6110a26dbbdc546c692933117f28ba/image-36.jpg)
![[OH ] Hydroxide Ion Concentration [OH ] Hydroxide Ion Concentration](https://slidetodoc.com/presentation_image_h/fa6110a26dbbdc546c692933117f28ba/image-37.jpg)

































- Slides: 72









$100 $100 $200 $200 $300 $300 $400 $400 $500 $500

What makes water an effective solvent?

Its Polar Nature

What characteristic of water remains the same no matter what is dissolved in it?

The ratio of hydrogen to oxygen

Hard water has a p. H higher than 7 and an abundance of calcium and magnesium salts. What would be the best cleaning solution for removing hard-water residue from drinking glasses?

A mild acid such as vinegar

Explain in detail what makes a water molecule polar. Double the score if correct!

Oxygen has greater electronegativity so it doesn’t share electrons equally, oxygen keeps the electrons most of the time

The picture shows the results of pouring a blue liquid into a clear liquid and allowing the mixture to settle for 25 minutes. Why did the solution end up looking like this?

2 Liquids are immiscible and the blue one has a higher density

What is an electrolyte?

A solution that conducts electricity.

What process is depicted in the following picture?

Dissociation

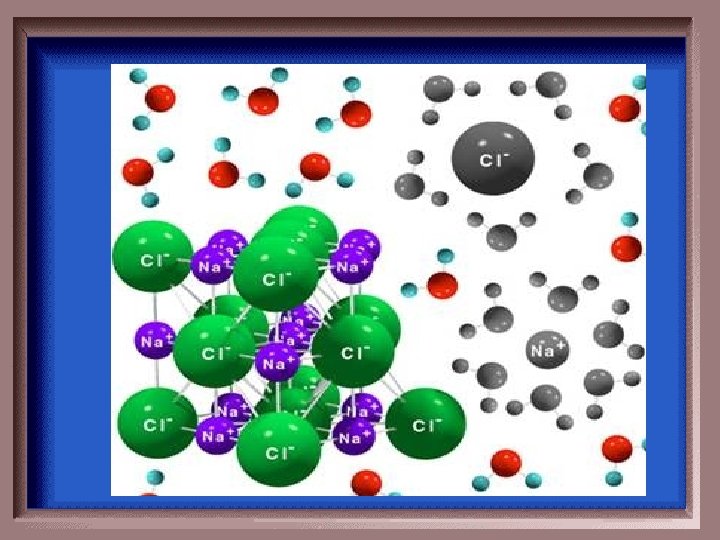
Why does dissolving salt in water increase the conductivity of the solution?

concentration of ions in the solution increases

Why will bathwater normally have electrolytic behaviors even though distilled water does not?

Bathwater generally contains dissolved minerals

Why doesn’t pure water conduct electricity?

No Ions or minerals are dissolved in it

A true solution is a homogeneous mixture and it has to also be _________

Colorless or transparent

What will be the p. H of a solution having an equal number of H+ ions and OH– ions?

p. H = 7
![What does the following symbol stand for H 3 O What does the following symbol stand for? [H 3 + O ]](https://slidetodoc.com/presentation_image_h/fa6110a26dbbdc546c692933117f28ba/image-34.jpg)
What does the following symbol stand for? [H 3 + O ]
![H 3 O Hydronium Ion Concentration [H 3 + O ] Hydronium Ion Concentration](https://slidetodoc.com/presentation_image_h/fa6110a26dbbdc546c692933117f28ba/image-35.jpg)
[H 3 + O ] Hydronium Ion Concentration
![What does the following symbol stand for OH What does the following symbol stand for? [OH ]](https://slidetodoc.com/presentation_image_h/fa6110a26dbbdc546c692933117f28ba/image-36.jpg)
What does the following symbol stand for? [OH ]
![OH Hydroxide Ion Concentration [OH ] Hydroxide Ion Concentration](https://slidetodoc.com/presentation_image_h/fa6110a26dbbdc546c692933117f28ba/image-37.jpg)
[OH ] Hydroxide Ion Concentration

Write down the 3 factors that influence the RATE of dissolving?

1. Temperature 2. Agitation / Stirring 3. Surface Area of Particles

Which choice has the most surface area? A or B

Choice B

Which of the following choices will dissolve more salt? 50˚ 100˚

For solids, as temperature increases solubility increases.

What can you do to liquid soda to get it to dissolve more carbonated gas (CO 2)?

Lower the Temperature

What does it mean to be miscible?

That substances are able to mix together

What are the 3 factors affecting solubility?

1. Nature of the Solvent 2. Pressure (gases only) 3. Temperature

Why would swimmers say that they can float more easily in the ocean than in a freshwater pond?

density of ocean water is higher than that of pond water

A block of maple wood with a volume of 405 cubic centimeters and a density of 0. 67 g/cm 3 is sawed in half. What is the density of one of the pieces?

The same: 0. 67 g/cm 3 Remember water always has a density of 1 g/cm 3 regardless whether you have a cup of water or a swimming pool full of water

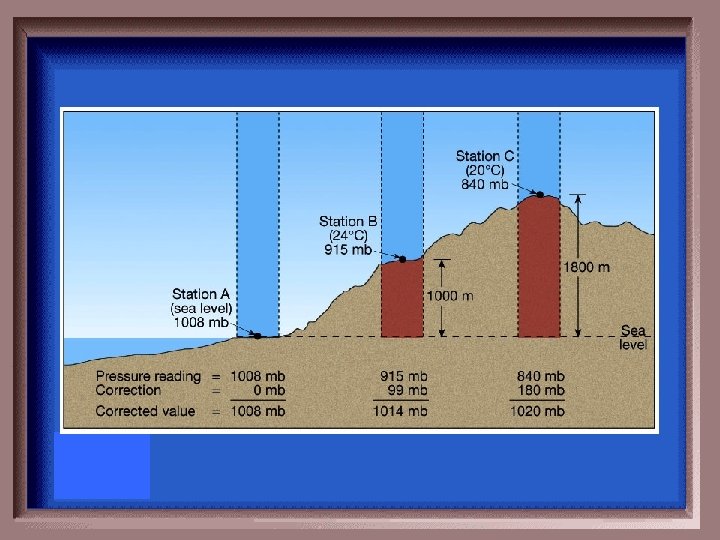
Water normally boils at 100°C at sea level and at 97°C on a North American mountaintop. Why?

Difference in air pressure

Students in a chemistry lab measure the time it takes four different 100 m. L solutions to pass through a hole in the bottom of a cup. Which of the following properties of the solutions is most likely being measured? A Buoyancy B Mass C Viscosity D Volume

C. Viscosity


Why are Bronsted. Lowry acids called proton donors?

+ H is the same thing as a proton.

What is a solvent?

Part of a solution that does the dissolving

What is a solute?

Part of a solution that gets dissolved in a solution by the solvent

Two clear solutions are placed in separate beakers. The first solution has a p. H of 4, and the p. H of the second solution is unknown. If the two solutions are mixed and the resulting p. H is 5, what can be said about the second solution?

Second solution had a higher concentration of OH – ions

Water molecules generally have which effect on a soluble ionic compound mixed into water?

They break the bonds between the ions.

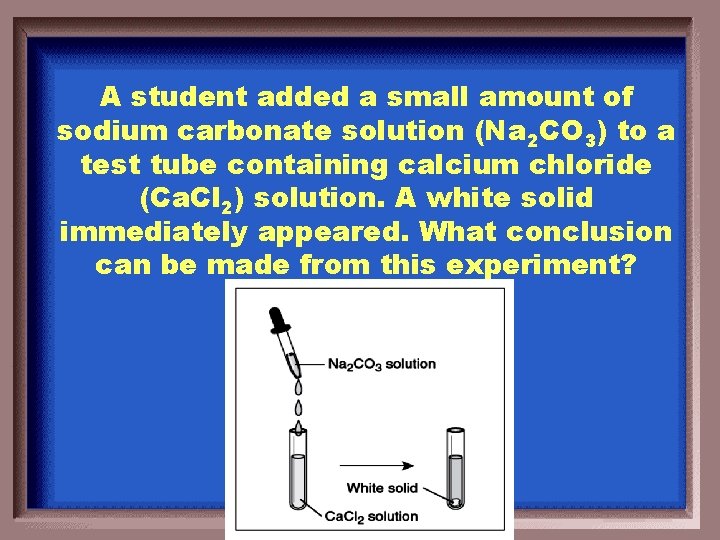
A student added a small amount of sodium carbonate solution (Na 2 CO 3) to a test tube containing calcium chloride (Ca. Cl 2) solution. A white solid immediately appeared. What conclusion can be made from this experiment?

At least one product of the reaction is insoluble.

How can you create your own Solubility curve for Sugar in water?

Find the amount of solute required for a solution to become saturated at various different temperatures
 400+400+400+200
400+400+400+200 300 + 200 + 200
300 + 200 + 200 100 200 300
100 200 300 200 + 200 + 300
200 + 200 + 300 100 100 100 100 100
100 100 100 100 100 100+200+300+400
100+200+300+400 100+200+300+400+500+600+700+800+900
100+200+300+400+500+600+700+800+900 100 + 200 + 300 + 400
100 + 200 + 300 + 400 100 200 300 400
100 200 300 400 100 200 300 400
100 200 300 400 100 200 300 400 500
100 200 300 400 500 100 200 300
100 200 300 200+200+100+100
200+200+100+100 400 + 300 + 300
400 + 300 + 300 300+300+400
300+300+400 300 300 400
300 300 400 300+300+400
300+300+400 300+300+400
300+300+400 300 square root
300 square root 300+300+400
300+300+400 200 300 400
200 300 400 200 300 300
200 300 300 200 + 200 + 300
200 + 200 + 300 400, 200, 100, 50, 25,
400, 200, 100, 50, 25, Testojack 200 reviews
Testojack 200 reviews 100+100=200
100+100=200 Who wrote this
Who wrote this The prime factorization of 88 is
The prime factorization of 88 is 200/400 meter training program
200/400 meter training program Sometimes between 200-300 b.c
Sometimes between 200-300 b.c 38 in yüzde 20'si kaçtır
38 in yüzde 20'si kaçtır Prime numbers 101 to 200
Prime numbers 101 to 200 Irla levels
Irla levels What's 200 * 300
What's 200 * 300 300 150 100 ekg
300 150 100 ekg Wandering atrial pacemaker
Wandering atrial pacemaker Gcf of 40
Gcf of 40 If the interval size is decreased from $200 to $100
If the interval size is decreased from $200 to $100 Qdx=200-2px qsx=-100+4px
Qdx=200-2px qsx=-100+4px Fractions equivalent to 100/200
Fractions equivalent to 100/200 Qdx=200-2px qsx=-100+4px
Qdx=200-2px qsx=-100+4px Gcse box plot questions
Gcse box plot questions Malloc lab 100/100
Malloc lab 100/100 C/100=f-32/180=k-273/100
C/100=f-32/180=k-273/100 1453-1337
1453-1337 What's 100 + 100
What's 100 + 100 100 iops/gb and 100,000 iops per volume oci
100 iops/gb and 100,000 iops per volume oci Numeros.romanos del 1 al 500
Numeros.romanos del 1 al 500 400 punkte abiturschnitt
400 punkte abiturschnitt Dimensional analysis chemistry definition
Dimensional analysis chemistry definition Complex incident
Complex incident Chapter 400
Chapter 400 18 numero romano
18 numero romano Qualcomm smart audio 400 platform
Qualcomm smart audio 400 platform Aqua troll 200 price
Aqua troll 200 price 586 400 000 in scientific notation
586 400 000 in scientific notation Dvgw arbeitsblatt w 400-2
Dvgw arbeitsblatt w 400-2 Trotec speedy 300 cena
Trotec speedy 300 cena Efavirenz 400 mg vs 600mg
Efavirenz 400 mg vs 600mg 600+400+500
600+400+500 Terminator t900
Terminator t900 Budesonide 400
Budesonide 400 Diketahui luas selimut suatu tabung 880 cm
Diketahui luas selimut suatu tabung 880 cm Jika luas juring aob 16cm2 maka luas juring boc adalah
Jika luas juring aob 16cm2 maka luas juring boc adalah Mavic 400 ai catheter
Mavic 400 ai catheter Giasion 400
Giasion 400 Quescom 400
Quescom 400 400 kg
400 kg 2500000/400
2500000/400 Trotec speedy 400
Trotec speedy 400 400 mm
400 mm Fototrofos
Fototrofos Crow 400 veiligheidsklasse
Crow 400 veiligheidsklasse