10 General Organic and Biochemistry 8 e Bettelheim
10 General, Organic, and Biochemistry, 8 e Bettelheim, Brown, Campbell, and Farrell © 2006 Thomson Learning, Inc. All rights reserved 10 -1
10 Chapter 10 Organic Chemistry © 2006 Thomson Learning, Inc. All rights reserved 10 -2
10 Organic Chemistry • Organic chemistry: the study of the compounds of carbon. • Organic compounds are made up of carbon and only a few other elements. • chief among these are hydrogen, oxygen, and nitrogen • also present are sulfur, phosphorus, and halogens (fluorine, chlorine, bromine, or iodine) © 2006 Thomson Learning, Inc. All rights reserved 10 -3
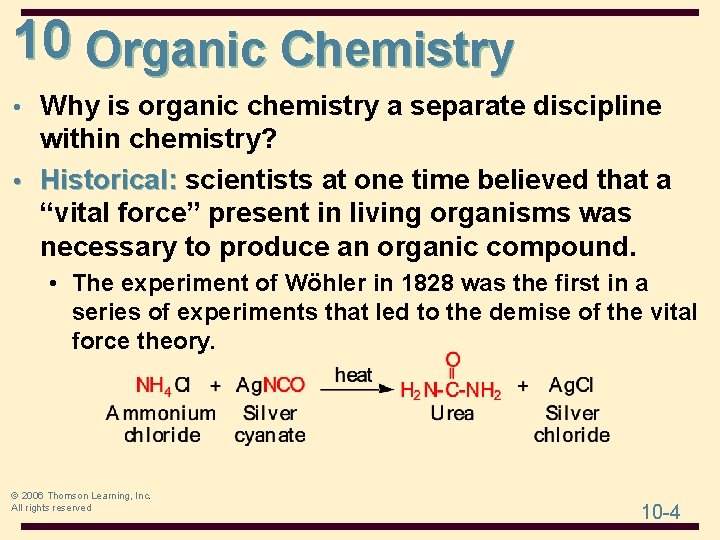
10 Organic Chemistry • Why is organic chemistry a separate discipline within chemistry? • Historical: scientists at one time believed that a “vital force” present in living organisms was necessary to produce an organic compound. • The experiment of Wöhler in 1828 was the first in a series of experiments that led to the demise of the vital force theory. © 2006 Thomson Learning, Inc. All rights reserved 10 -4
10 Organic Chemistry • The sheer number of organic compounds • Chemists have discovered or made over 10 million organic compounds and an estimated 100, 000 new ones are discovered or made each year. • By comparison, chemists have discovered or made an estimated 1. 7 million inorganic compounds. • Thus, approximately 85% of all known compounds are organic. • The link to biochemistry • Carbohydrates, lipids, proteins, enzymes, nucleic acids, hormones, vitamins, and almost all other chemicals in living systems are organic compounds. © 2006 Thomson Learning, Inc. All rights reserved 10 -5
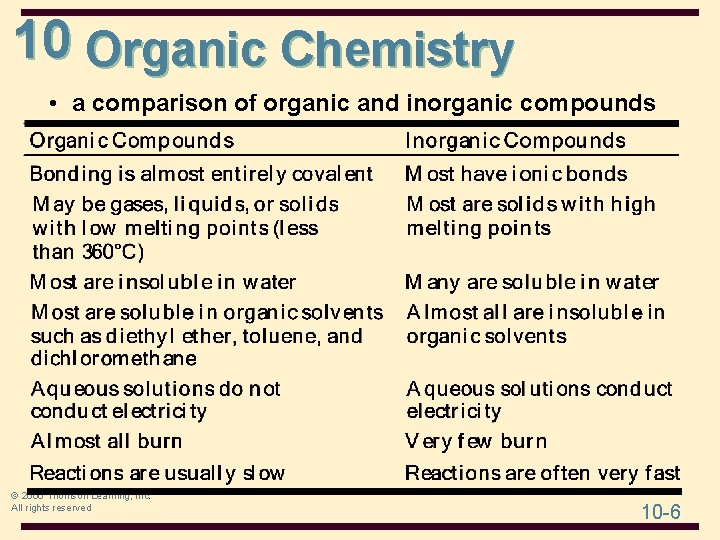
10 Organic Chemistry • a comparison of organic and inorganic compounds © 2006 Thomson Learning, Inc. All rights reserved 10 -6
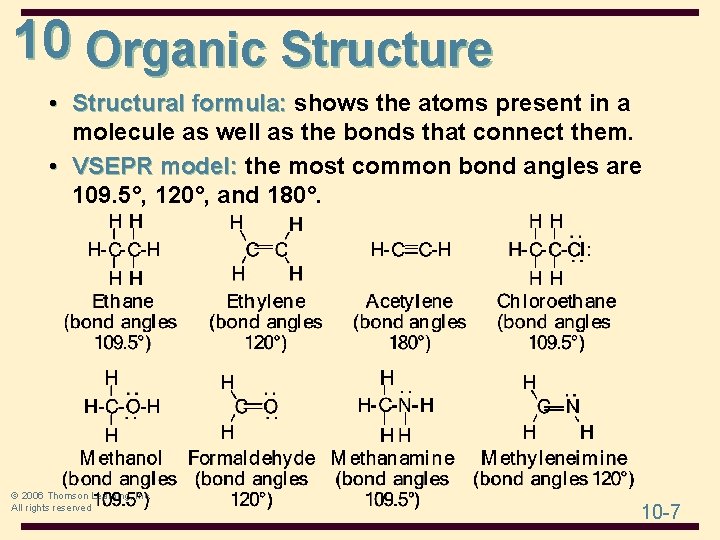
10 Organic Structure • Structural formula: shows the atoms present in a molecule as well as the bonds that connect them. • VSEPR model: the most common bond angles are 109. 5°, 120°, and 180°. © 2006 Thomson Learning, Inc. All rights reserved 10 -7
10 Organic Structure • Among neutral (uncharged) organic compounds: • Carbon: normally forms four covalent bonds and has no unshared pairs of electrons. • Hydrogen: forms one covalent bond and no unshared pairs of electrons. • Nitrogen: normally forms three covalent bonds and has one unshared pair of electrons. • Oxygen: normally forms two covalent bonds and has two unshared pairs of electrons. • Halogen: normally forms one covalent bond and has three unshared pairs of electrons. © 2006 Thomson Learning, Inc. All rights reserved 10 -8
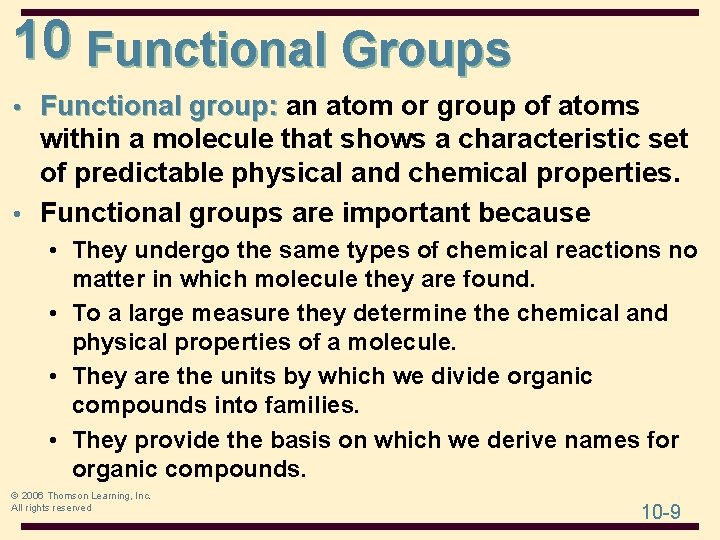
10 Functional Groups • Functional group: an atom or group of atoms within a molecule that shows a characteristic set of predictable physical and chemical properties. • Functional groups are important because • They undergo the same types of chemical reactions no matter in which molecule they are found. • To a large measure they determine the chemical and physical properties of a molecule. • They are the units by which we divide organic compounds into families. • They provide the basis on which we derive names for organic compounds. © 2006 Thomson Learning, Inc. All rights reserved 10 -9
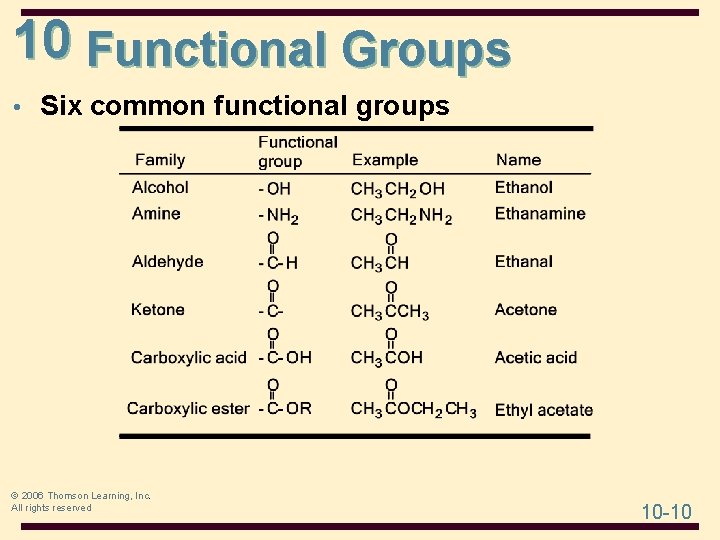
10 Functional Groups • Six common functional groups © 2006 Thomson Learning, Inc. All rights reserved 10 -10
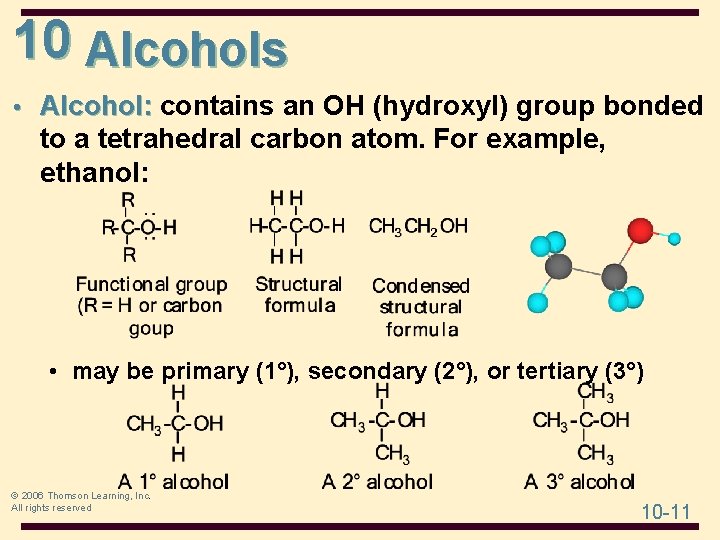
10 Alcohols • Alcohol: contains an OH (hydroxyl) group bonded to a tetrahedral carbon atom. For example, ethanol: • may be primary (1°), secondary (2°), or tertiary (3°) © 2006 Thomson Learning, Inc. All rights reserved 10 -11

10 Alcohols • Problem: draw Lewis structures and condensed structural formulas for the two alcohols with the molecular formula C 3 H 8 O. © 2006 Thomson Learning, Inc. All rights reserved 10 -12
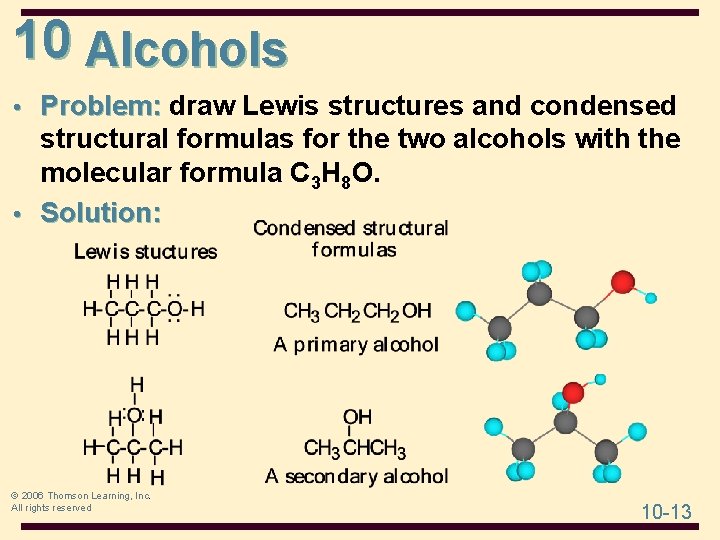
10 Alcohols • Problem: draw Lewis structures and condensed structural formulas for the two alcohols with the molecular formula C 3 H 8 O. • Solution: © 2006 Thomson Learning, Inc. All rights reserved 10 -13
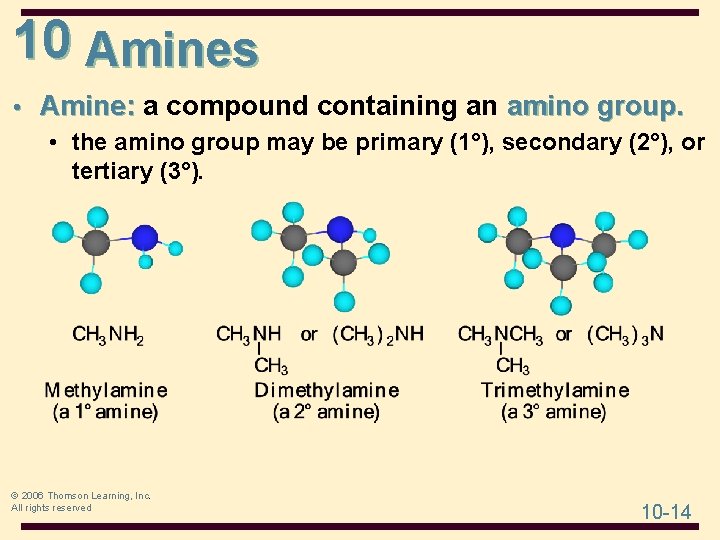
10 Amines • Amine: a compound containing an amino group. • the amino group may be primary (1°), secondary (2°), or tertiary (3°). © 2006 Thomson Learning, Inc. All rights reserved 10 -14
10 Amines • Problem: draw condensed structural formulas for the two primary amines with the molecular formula C 3 H 9 N. © 2006 Thomson Learning, Inc. All rights reserved 10 -15
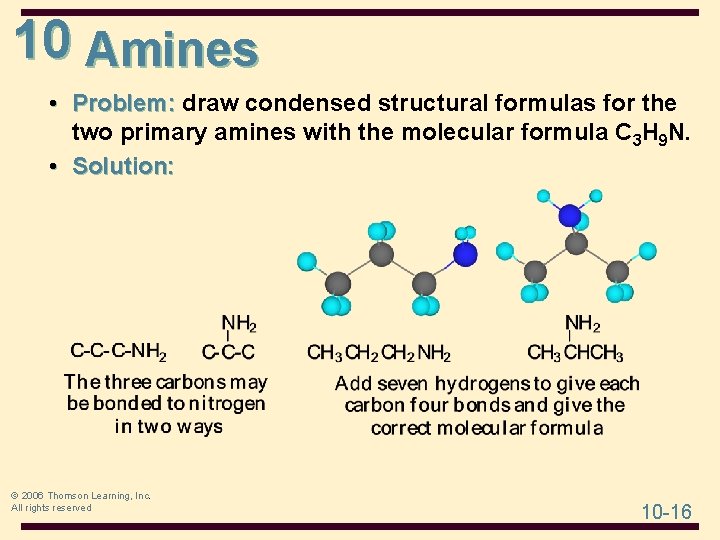
10 Amines • Problem: draw condensed structural formulas for the two primary amines with the molecular formula C 3 H 9 N. • Solution: © 2006 Thomson Learning, Inc. All rights reserved 10 -16
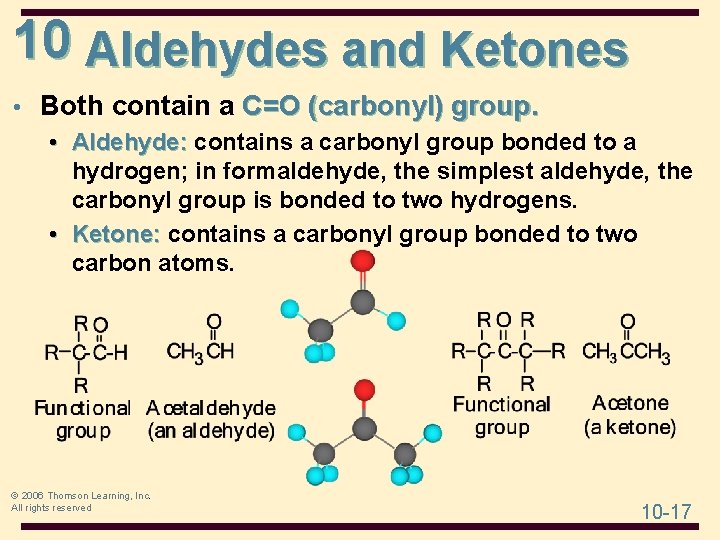
10 Aldehydes and Ketones • Both contain a C=O (carbonyl) group. • Aldehyde: contains a carbonyl group bonded to a hydrogen; in formaldehyde, the simplest aldehyde, the carbonyl group is bonded to two hydrogens. • Ketone: contains a carbonyl group bonded to two carbon atoms. © 2006 Thomson Learning, Inc. All rights reserved 10 -17
10 Aldehydes and Ketones • Problem: draw condensed structural formulas for the two aldehydes with the molecular formula C 4 H 8 O. © 2006 Thomson Learning, Inc. All rights reserved 10 -18
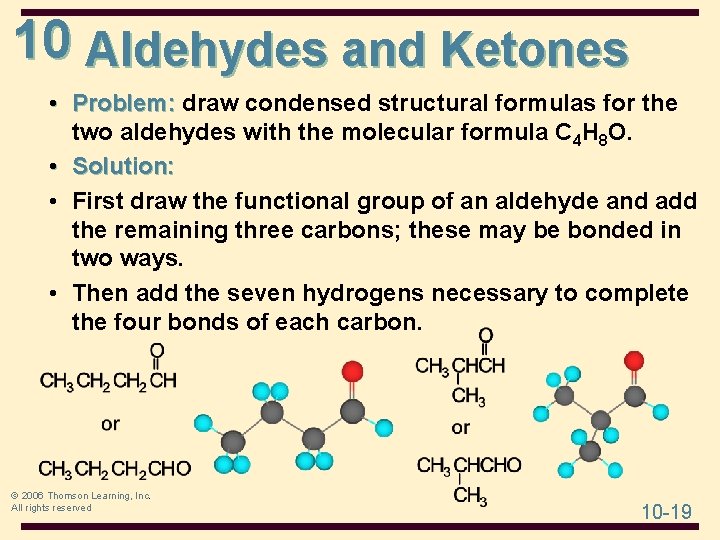
10 Aldehydes and Ketones • Problem: draw condensed structural formulas for the two aldehydes with the molecular formula C 4 H 8 O. • Solution: • First draw the functional group of an aldehyde and add the remaining three carbons; these may be bonded in two ways. • Then add the seven hydrogens necessary to complete the four bonds of each carbon. © 2006 Thomson Learning, Inc. All rights reserved 10 -19
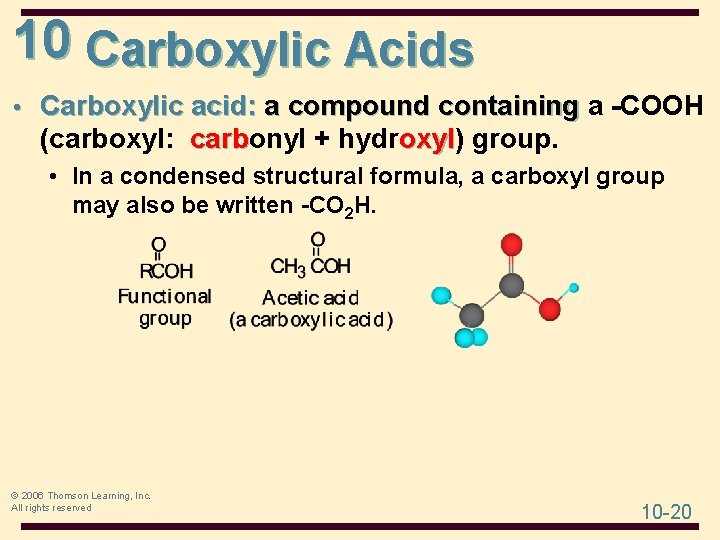
10 Carboxylic Acids • Carboxylic acid: a compound containing a -COOH (carboxyl: carbonyl + hydroxyl) carb oxyl group. • In a condensed structural formula, a carboxyl group may also be written -CO 2 H. © 2006 Thomson Learning, Inc. All rights reserved 10 -20
10 Carboxylic Acids • Problem: draw a condensed structural formula for the single carboxylic acid with the molecular formula C 3 H 6 O 2 © 2006 Thomson Learning, Inc. All rights reserved 10 -21
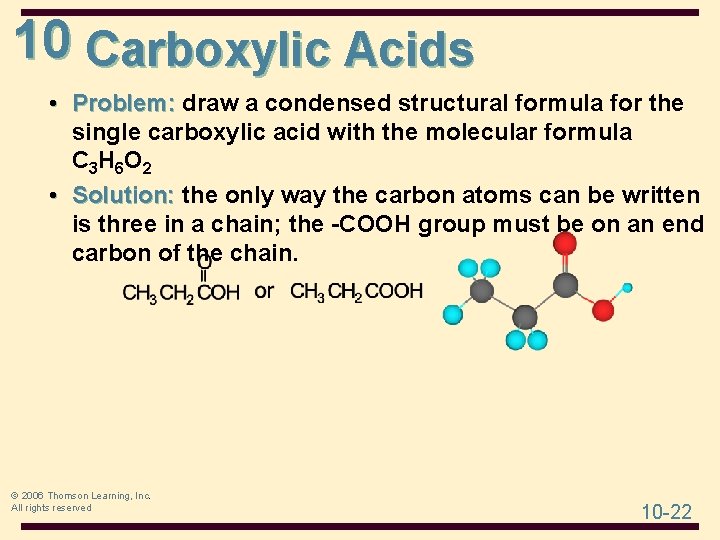
10 Carboxylic Acids • Problem: draw a condensed structural formula for the single carboxylic acid with the molecular formula C 3 H 6 O 2 • Solution: the only way the carbon atoms can be written is three in a chain; the -COOH group must be on an end carbon of the chain. © 2006 Thomson Learning, Inc. All rights reserved 10 -22
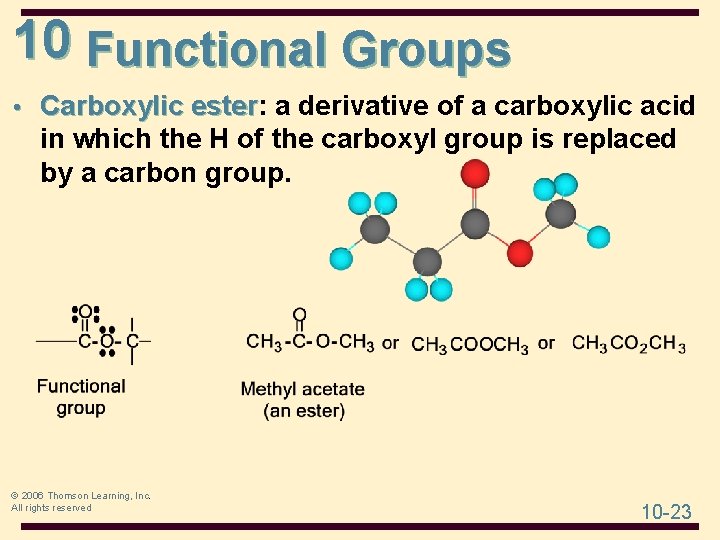
10 Functional Groups • Carboxylic ester: ester a derivative of a carboxylic acid in which the H of the carboxyl group is replaced by a carbon group. © 2006 Thomson Learning, Inc. All rights reserved 10 -23
10 Chapter 10 Organic Chemistry End Chapter 10 © 2006 Thomson Learning, Inc. All rights reserved 10 -24
- Slides: 24