10 Alkyl Halides What Is an Alkyl Halide





- Slides: 24

10. Alkyl Halides

What Is an Alkyl Halide n An organic compound containing at least one carbon- halogen bond (C-X) n X (F, Cl, Br, I) replaces H n Can contain many C-X bonds n Properties and some uses n Fire-resistant solvents n Refrigerants n Pharmaceuticals and precursors 2

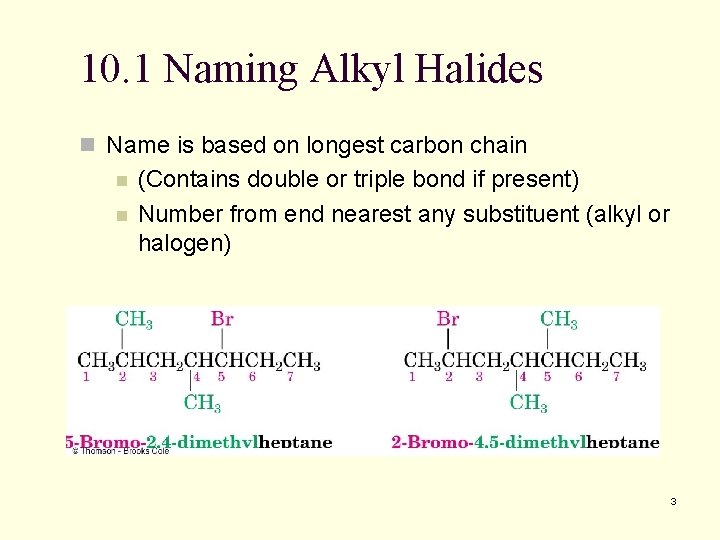
10. 1 Naming Alkyl Halides n Name is based on longest carbon chain n n (Contains double or triple bond if present) Number from end nearest any substituent (alkyl or halogen) 3

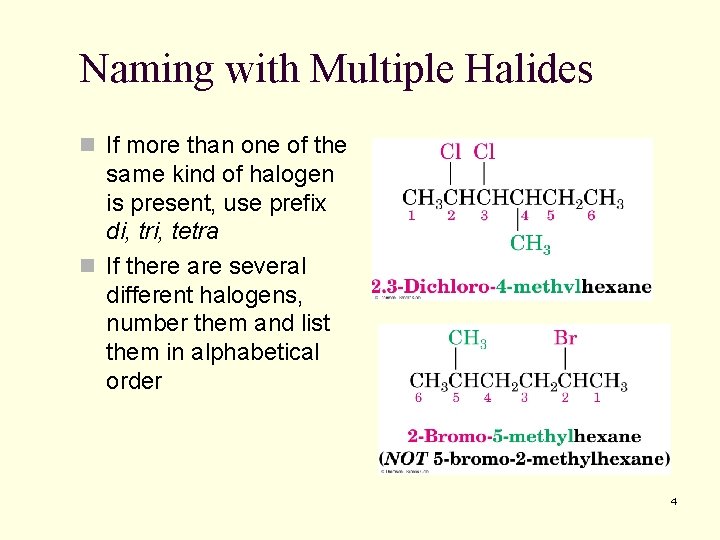
Naming with Multiple Halides n If more than one of the same kind of halogen is present, use prefix di, tri, tetra n If there are several different halogens, number them and list them in alphabetical order 4

Naming if Two Halides or Alkyl Are Equally Distant from Ends of Chain n Begin at the end nearer the substituent whose name comes first in the alphabet 5

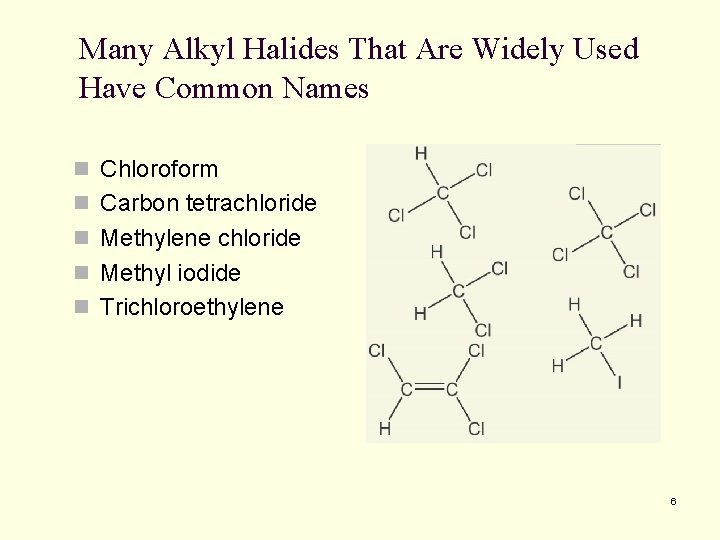
Many Alkyl Halides That Are Widely Used Have Common Names n Chloroform n Carbon tetrachloride n Methylene chloride n Methyl iodide n Trichloroethylene 6

10. 2 Structure of Alkyl Halides n C-X bond is longer as you go down periodic table n C-X bond is weaker as you go down periodic table n C-X bond is polarized with slight positive on carbon and slight negative on halogen 7

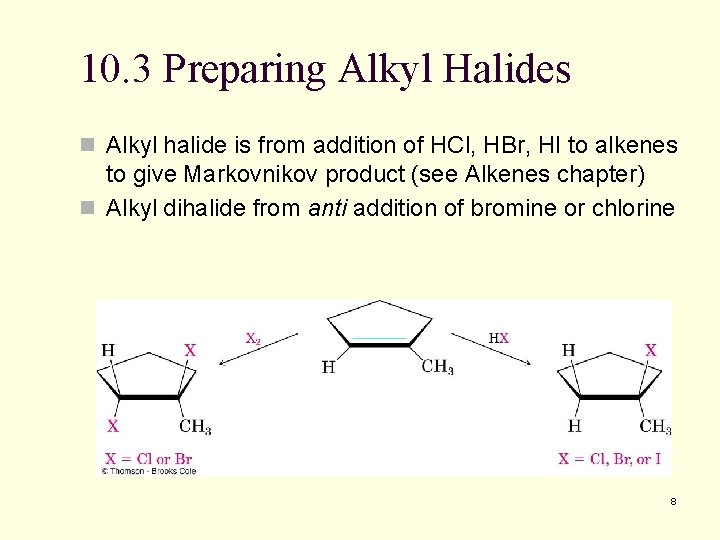
10. 3 Preparing Alkyl Halides n Alkyl halide is from addition of HCl, HBr, HI to alkenes to give Markovnikov product (see Alkenes chapter) n Alkyl dihalide from anti addition of bromine or chlorine 8

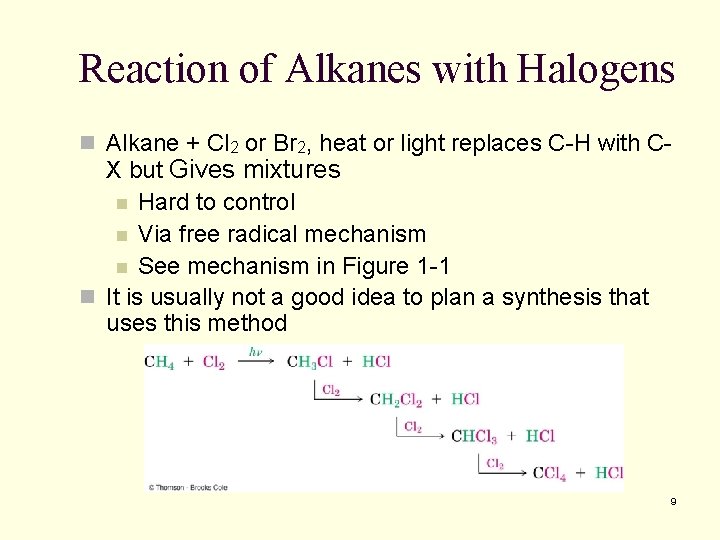
Reaction of Alkanes with Halogens n Alkane + Cl 2 or Br 2, heat or light replaces C-H with C- X but Gives mixtures n Hard to control n Via free radical mechanism n See mechanism in Figure 1 -1 n It is usually not a good idea to plan a synthesis that uses this method 9

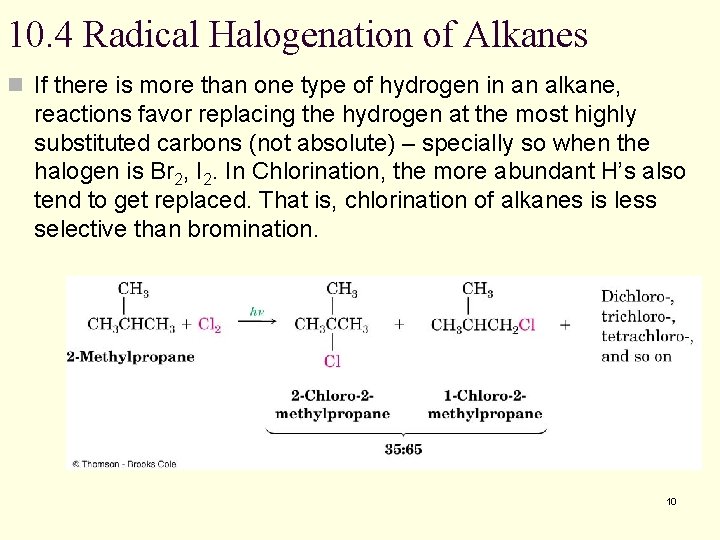
10. 4 Radical Halogenation of Alkanes n If there is more than one type of hydrogen in an alkane, reactions favor replacing the hydrogen at the most highly substituted carbons (not absolute) – specially so when the halogen is Br 2, I 2. In Chlorination, the more abundant H’s also tend to get replaced. That is, chlorination of alkanes is less selective than bromination. 10

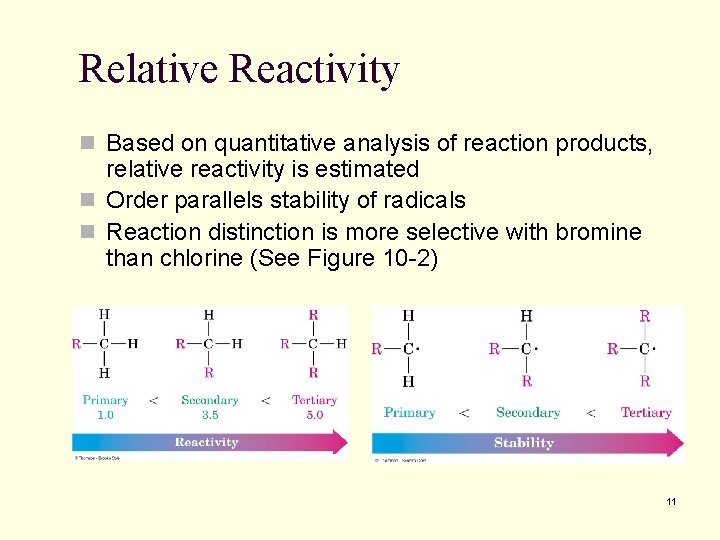
Relative Reactivity n Based on quantitative analysis of reaction products, relative reactivity is estimated n Order parallels stability of radicals n Reaction distinction is more selective with bromine than chlorine (See Figure 10 -2) 11

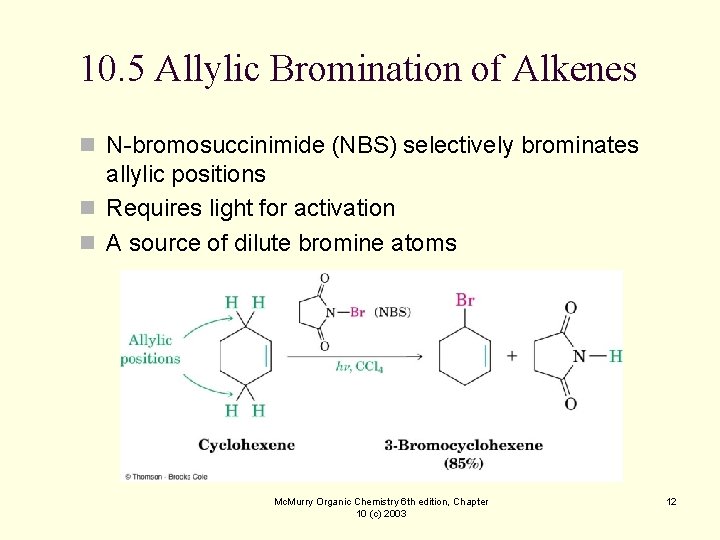
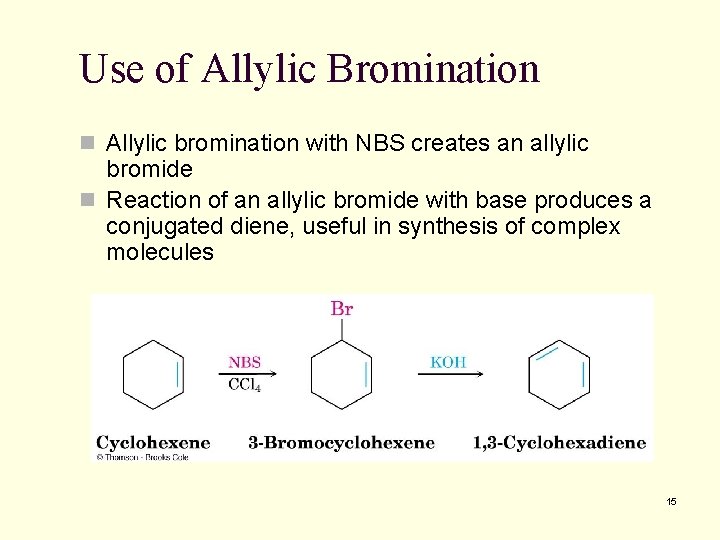
10. 5 Allylic Bromination of Alkenes n N-bromosuccinimide (NBS) selectively brominates allylic positions n Requires light for activation n A source of dilute bromine atoms Mc. Murry Organic Chemistry 6 th edition, Chapter 10 (c) 2003 12

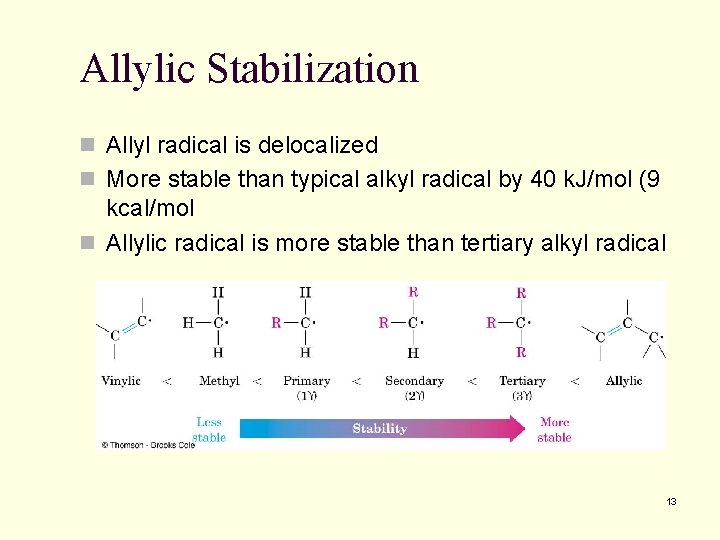
Allylic Stabilization n Allyl radical is delocalized n More stable than typical alkyl radical by 40 k. J/mol (9 kcal/mol n Allylic radical is more stable than tertiary alkyl radical 13

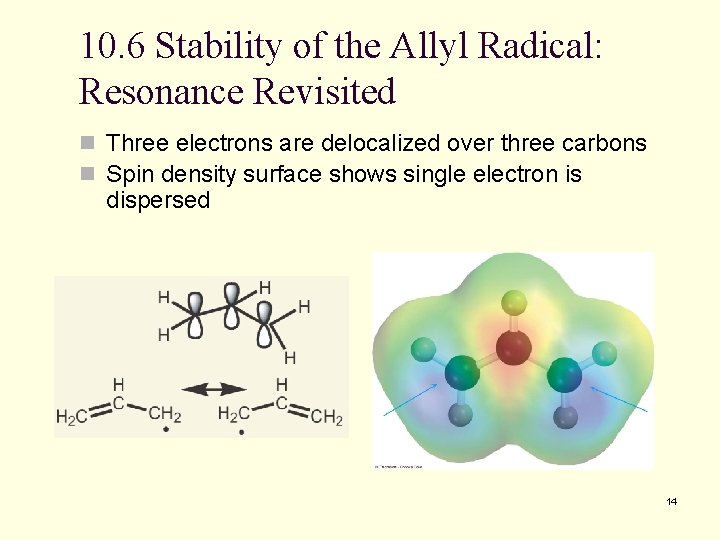
10. 6 Stability of the Allyl Radical: Resonance Revisited n Three electrons are delocalized over three carbons n Spin density surface shows single electron is dispersed 14

Use of Allylic Bromination n Allylic bromination with NBS creates an allylic bromide n Reaction of an allylic bromide with base produces a conjugated diene, useful in synthesis of complex molecules 15

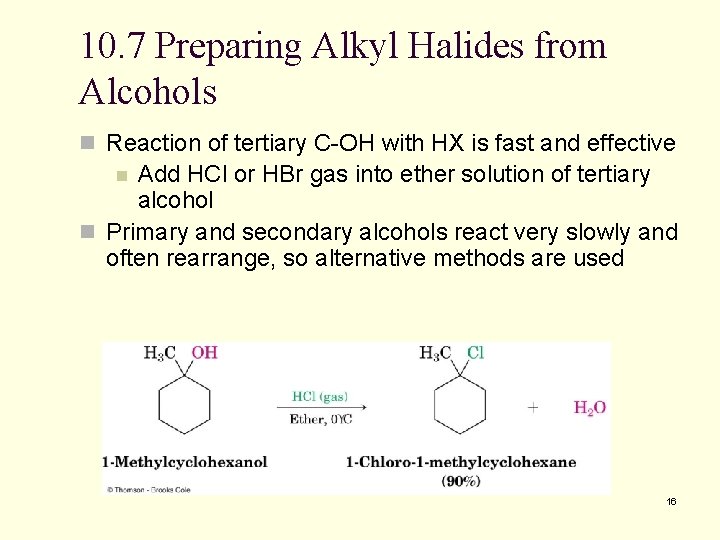
10. 7 Preparing Alkyl Halides from Alcohols n Reaction of tertiary C-OH with HX is fast and effective Add HCl or HBr gas into ether solution of tertiary alcohol n Primary and secondary alcohols react very slowly and often rearrange, so alternative methods are used n 16

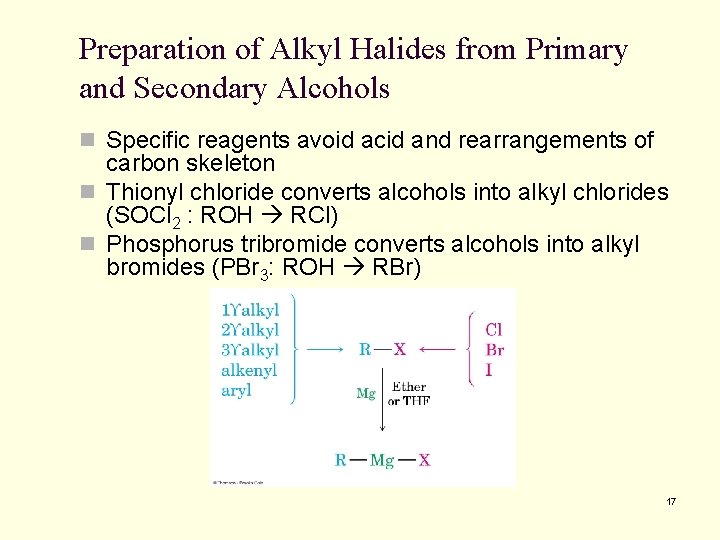
Preparation of Alkyl Halides from Primary and Secondary Alcohols n Specific reagents avoid acid and rearrangements of carbon skeleton n Thionyl chloride converts alcohols into alkyl chlorides (SOCl 2 : ROH RCl) n Phosphorus tribromide converts alcohols into alkyl bromides (PBr 3: ROH RBr) 17

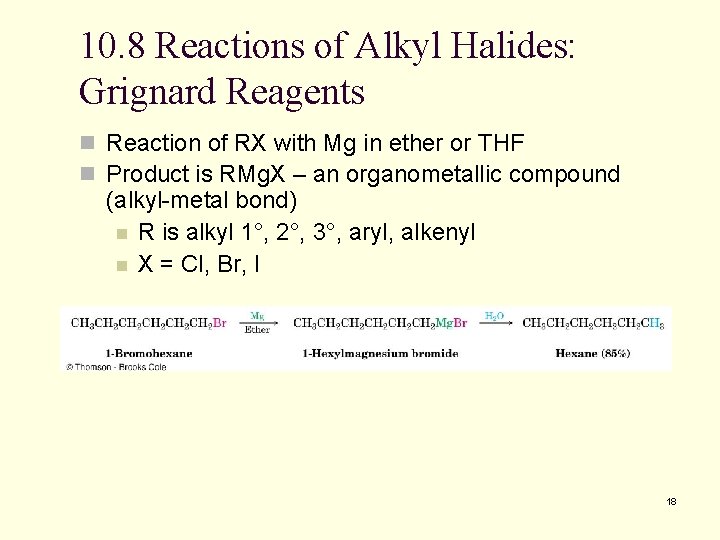
10. 8 Reactions of Alkyl Halides: Grignard Reagents n Reaction of RX with Mg in ether or THF n Product is RMg. X – an organometallic compound (alkyl-metal bond) n R is alkyl 1°, 2°, 3°, aryl, alkenyl n X = Cl, Br, I 18

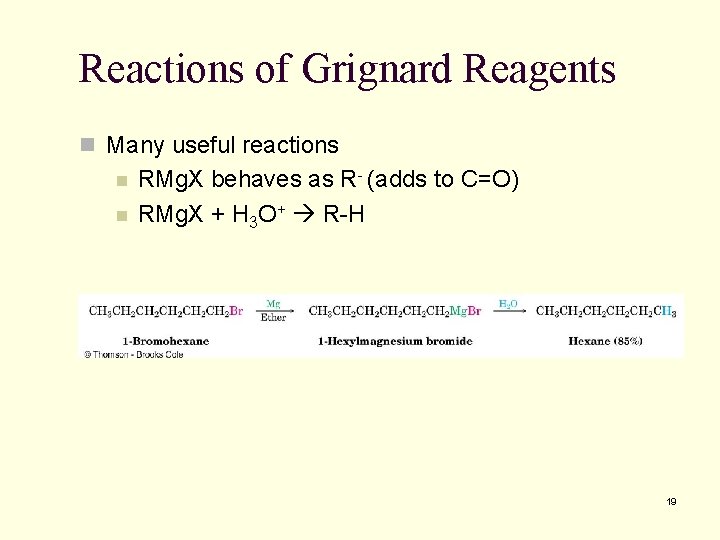
Reactions of Grignard Reagents n Many useful reactions n n RMg. X behaves as R- (adds to C=O) RMg. X + H 3 O+ R-H 19

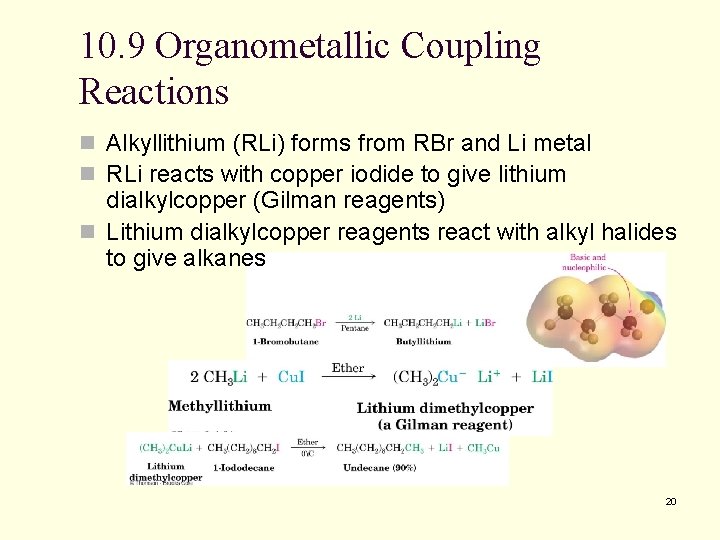
10. 9 Organometallic Coupling Reactions n Alkyllithium (RLi) forms from RBr and Li metal n RLi reacts with copper iodide to give lithium dialkylcopper (Gilman reagents) n Lithium dialkylcopper reagents react with alkyl halides to give alkanes 20

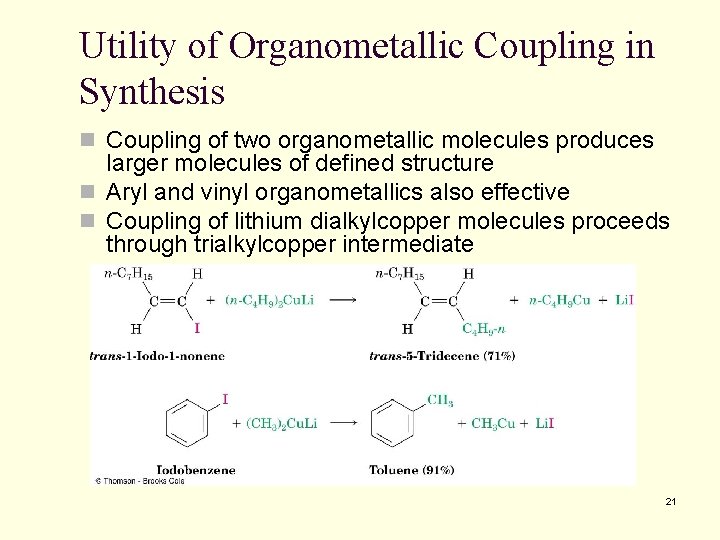
Utility of Organometallic Coupling in Synthesis n Coupling of two organometallic molecules produces larger molecules of defined structure n Aryl and vinyl organometallics also effective n Coupling of lithium dialkylcopper molecules proceeds through trialkylcopper intermediate 21

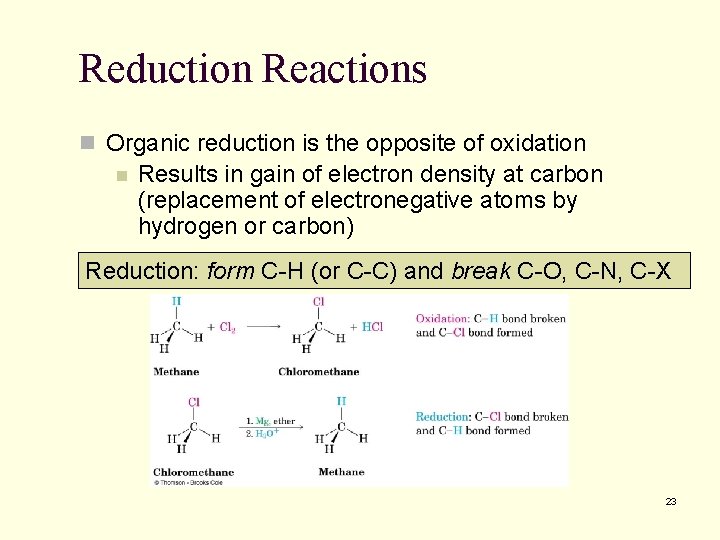
10. 10 Oxidation and Reduction in Organic Chemistry n In organic chemistry, we say that oxidation occurs when a carbon or hydrogen that is connected to a carbon atom in a structure is replaced by oxygen, nitrogen, or halogen n Not defined as loss of electrons by an atom as in inorganic chemistry n Oxidation is a reaction that results in loss of electron density at carbon (as more electronegative atoms replace hydrogen or carbon) Oxidation: break C-H (or C-C) and form C-O, C -N, C-X 22

Reduction Reactions n Organic reduction is the opposite of oxidation n Results in gain of electron density at carbon (replacement of electronegative atoms by hydrogen or carbon) Reduction: form C-H (or C-C) and break C-O, C-N, C-X 23

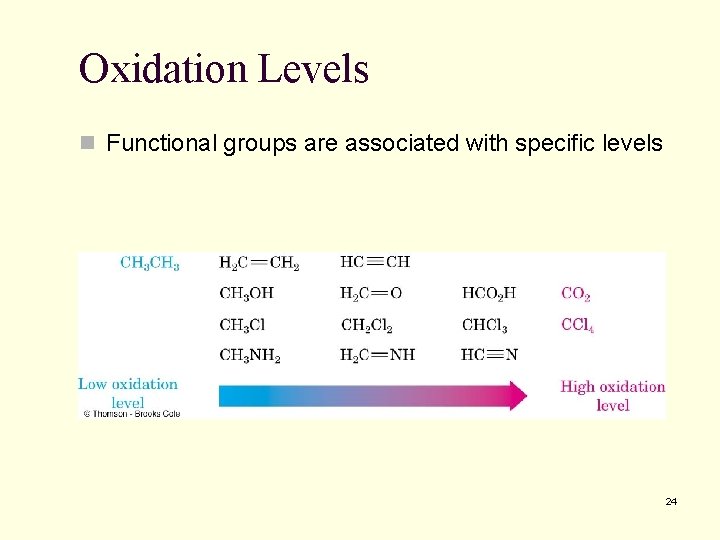
Oxidation Levels n Functional groups are associated with specific levels 24