10 7 Shifting the Equilibrium Position LO I

10. 7 Shifting the Equilibrium Position LO: I understand how the equilibrium position can be changed.
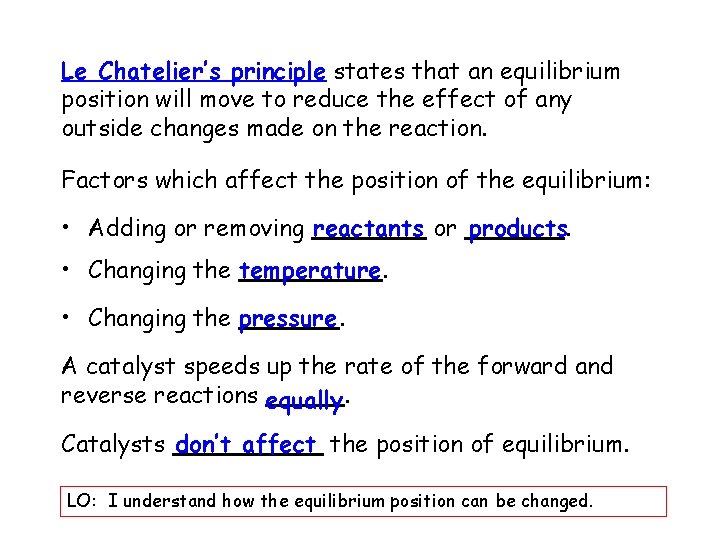
Le Chatelier’s principle states that an equilibrium position will move to reduce the effect of any outside changes made on the reaction. Factors which affect the position of the equilibrium: • Adding or removing reactants or products. • Changing the temperature. • Changing the pressure. A catalyst speeds up the rate of the forward and reverse reactions equally. Catalysts don’t affect the position of equilibrium. LO: I understand how the equilibrium position can be changed.
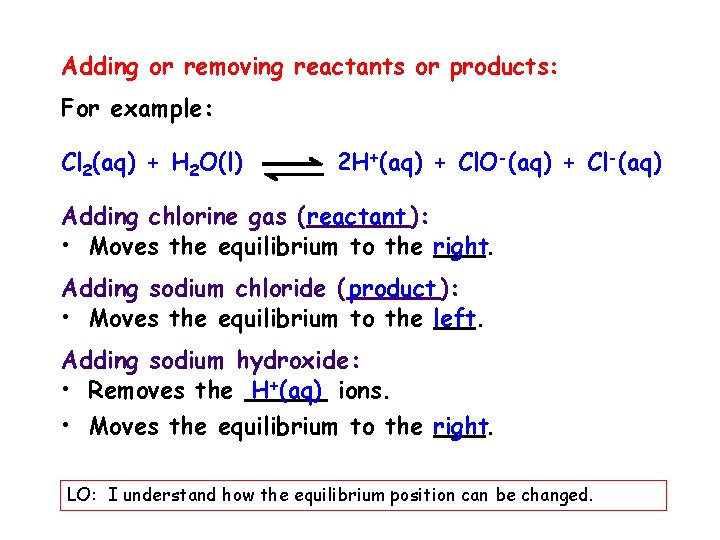
Adding or removing reactants or products: For example: Cl 2(aq) + H 2 O(l) 2 H+(aq) + Cl. O-(aq) + Cl-(aq) Adding chlorine gas ( reactant ): • Moves the equilibrium to the right. Adding sodium chloride ( product ): • Moves the equilibrium to the left. Adding sodium hydroxide: • Removes the H+(aq) ions. • Moves the equilibrium to the right. LO: I understand how the equilibrium position can be changed.
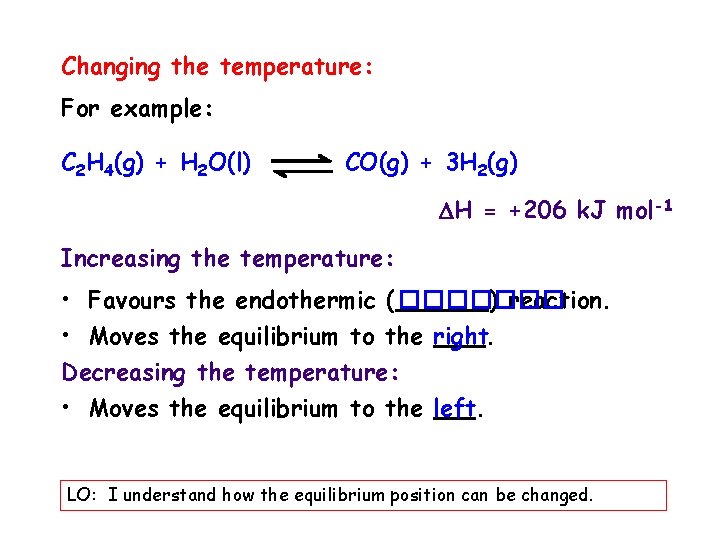
Changing the temperature: For example: C 2 H 4(g) + H 2 O(l) CO(g) + 3 H 2(g) H = +206 k. J mol-1 Increasing the temperature: • Favours the endothermic ( ������� ) reaction. • Moves the equilibrium to the right. Decreasing the temperature: • Moves the equilibrium to the left. LO: I understand how the equilibrium position can be changed.
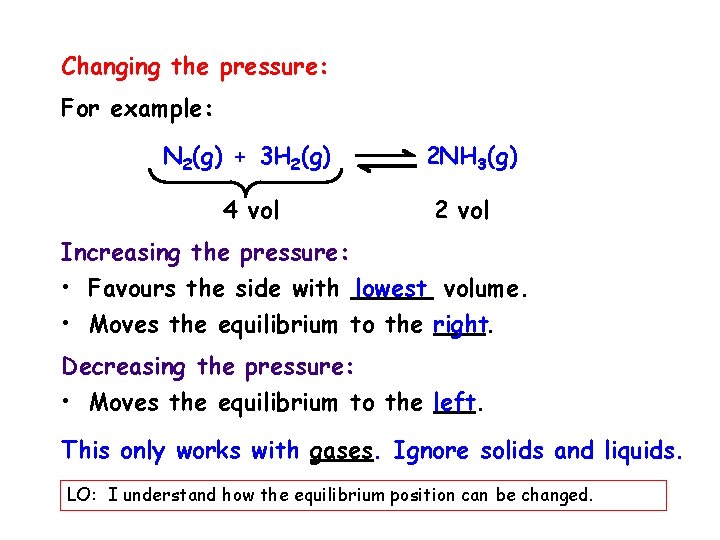
Changing the pressure: For example: N 2(g) + 3 H 2(g) 4 vol 2 NH 3(g) 2 vol Increasing the pressure: • Favours the side with lowest volume. • Moves the equilibrium to the right. Decreasing the pressure: • Moves the equilibrium to the left. This only works with gases. Ignore solids and liquids. LO: I understand how the equilibrium position can be changed.
- Slides: 5