10 5 Classes of Dienes Classification of Dienes











- Slides: 31

10. 5 Classes of Dienes

Classification of Dienes isolated diene conjugated diene C cumulated diene

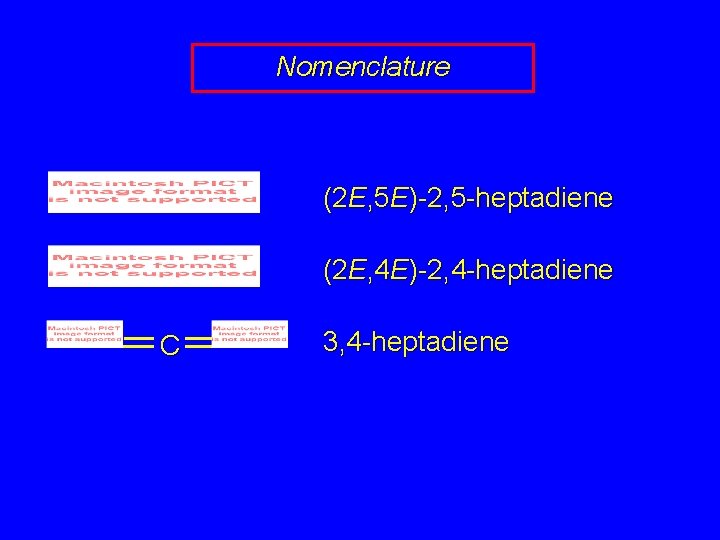
Nomenclature (2 E, 5 E)-2, 5 -heptadiene (2 E, 4 E)-2, 4 -heptadiene C 3, 4 -heptadiene

10. 6 Relative Stabilities of Dienes

Heats of Hydrogenation 252 k. J/mol 1, 3 -pentadiene is 26 k. J/mol more stable than 1, 4 -pentadiene, but some of this stabilization is because it also contains a more highly substituted double bond 226 k. J/mol

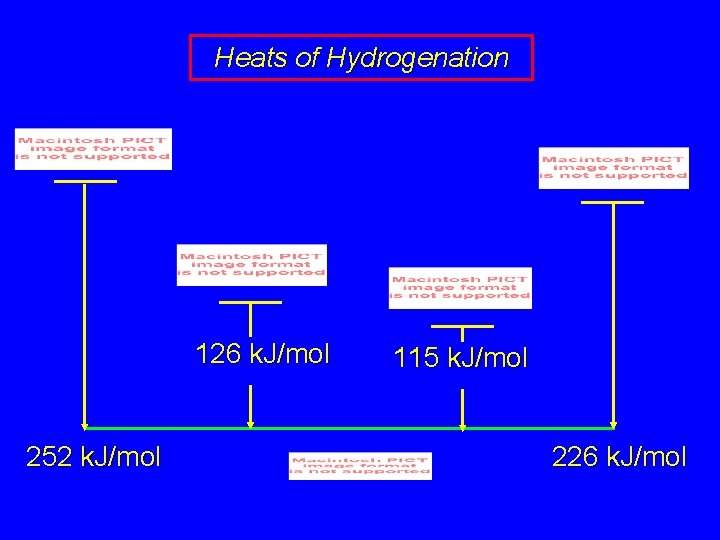
Heats of Hydrogenation 126 k. J/mol 252 k. J/mol 115 k. J/mol 226 k. J/mol

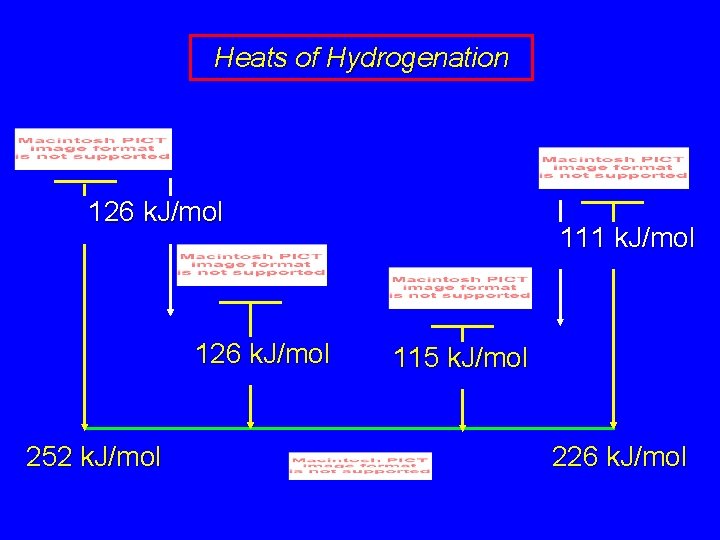
Heats of Hydrogenation 126 k. J/mol 252 k. J/mol 111 k. J/mol 115 k. J/mol 226 k. J/mol

Heats of Hydrogenation 126 k. J/mol 111 k. J/mol when terminal double bond is conjugated with other double bond, its heat of hydrogenation is 15 k. J/mol less than when isolated

Heats of Hydrogenation 126 k. J/mol 111 k. J/mol this extra 15 k. J/mol is known by several terms stabilization energy delocalization energy resonance energy

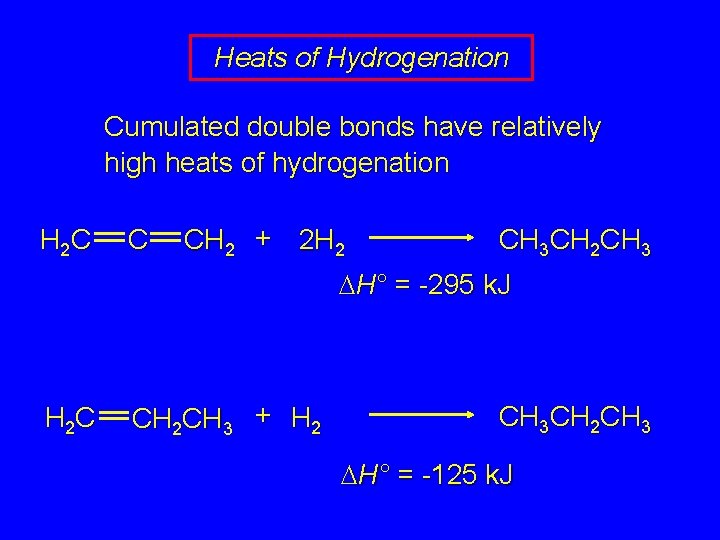
Heats of Hydrogenation Cumulated double bonds have relatively high heats of hydrogenation H 2 C C CH 2 + 2 H 2 CH 3 CH 2 CH 3 DH° = -295 k. J H 2 C CH 2 CH 3 + H 2 CH 3 CH 2 CH 3 DH° = -125 k. J

10. 7 Bonding in Conjugated Dienes

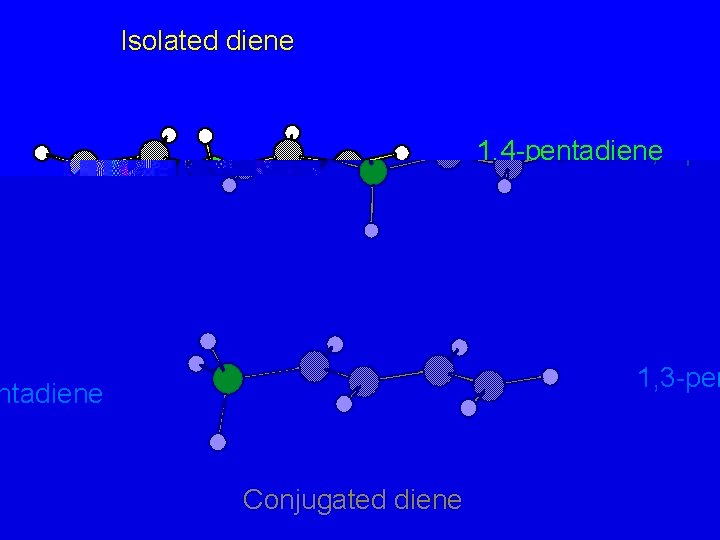
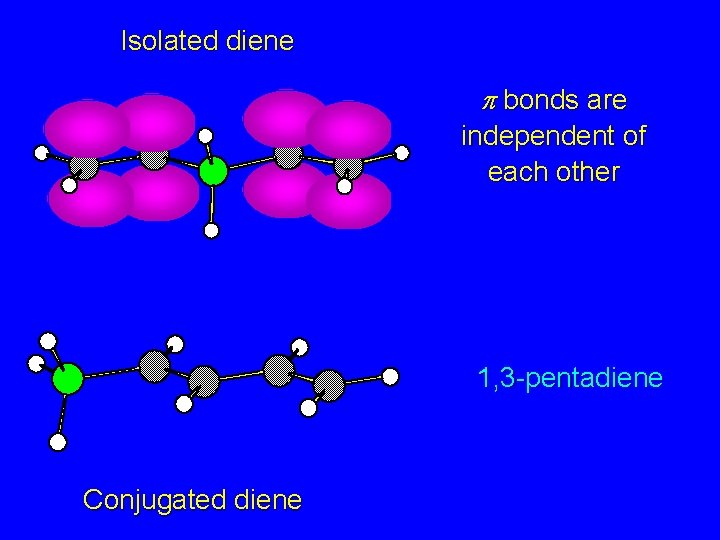
Isolated diene 1, 4 -pentadiene 1, 3 -pentadiene Conjugated diene

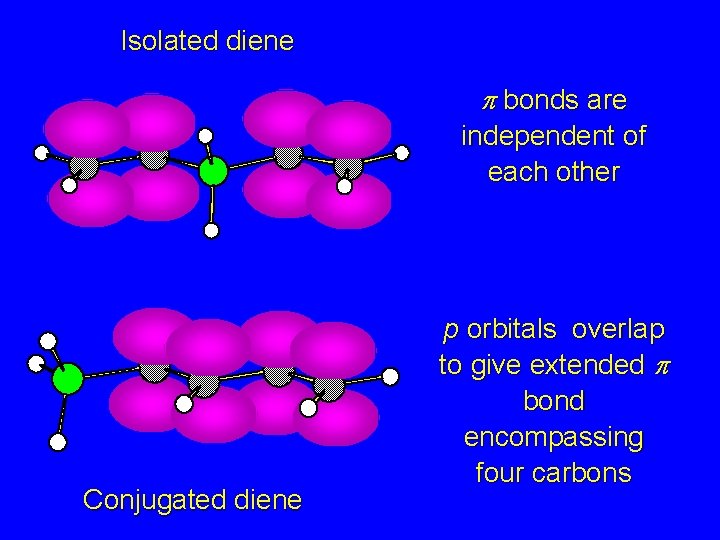
Isolated diene p bonds are independent of each other 1, 3 -pentadiene Conjugated diene

Isolated diene p bonds are independent of each other Conjugated diene p orbitals overlap to give extended p bond encompassing four carbons

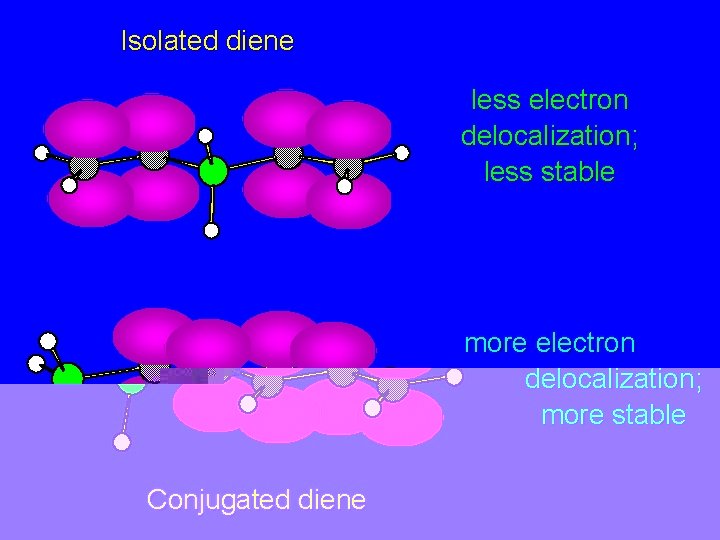
Isolated diene less electron delocalization; less stable more electron delocalization; more stable Conjugated diene

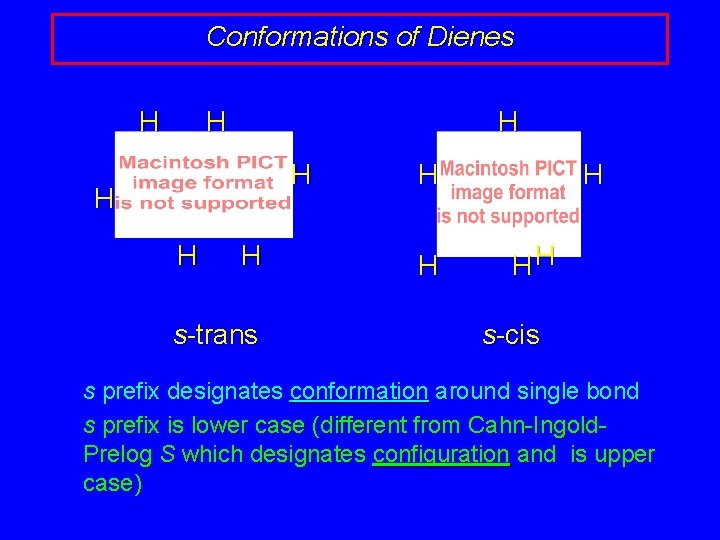
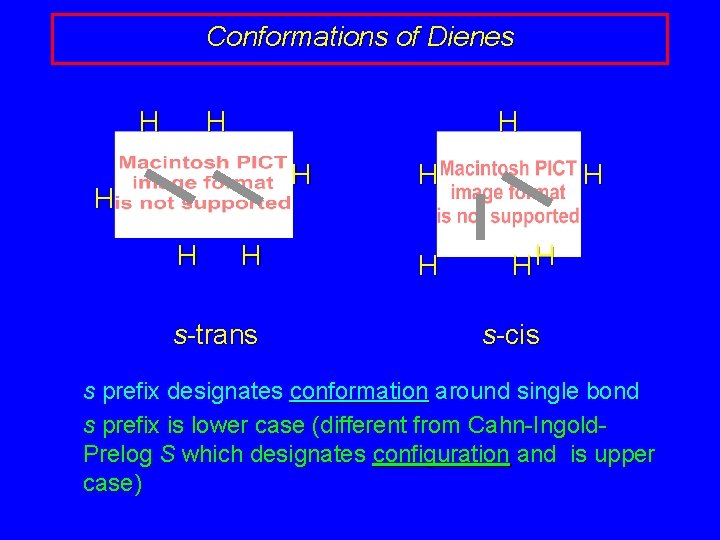
Conformations of Dienes H H H H s-trans H HH s-cis s prefix designates conformation around single bond s prefix is lower case (different from Cahn-Ingold. Prelog S which designates configuration and is upper case)

Conformations of Dienes H H H H s-trans H HH s-cis s prefix designates conformation around single bond s prefix is lower case (different from Cahn-Ingold. Prelog S which designates configuration and is upper case)

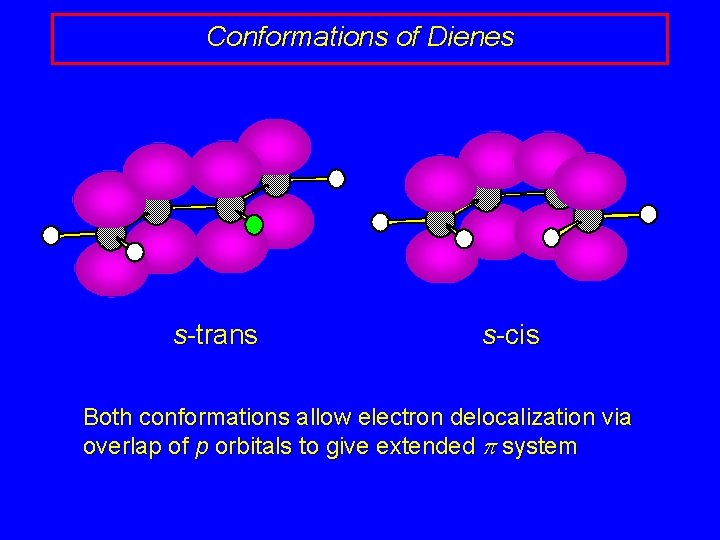
Conformations of Dienes s-trans s-cis Both conformations allow electron delocalization via overlap of p orbitals to give extended p system

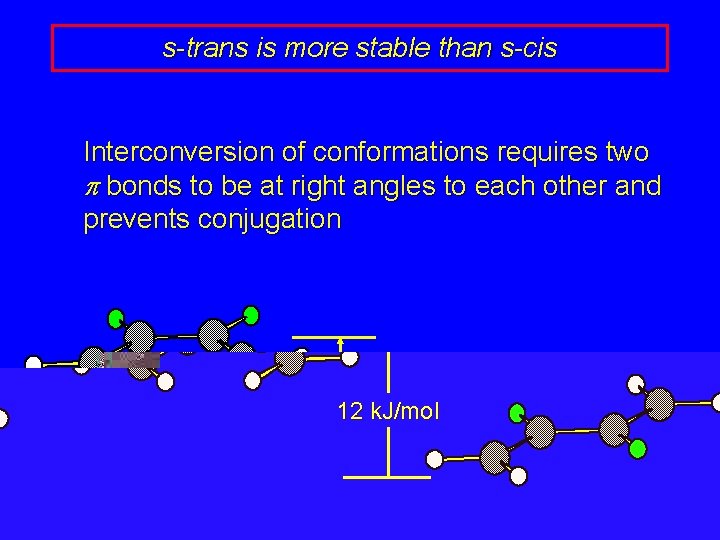
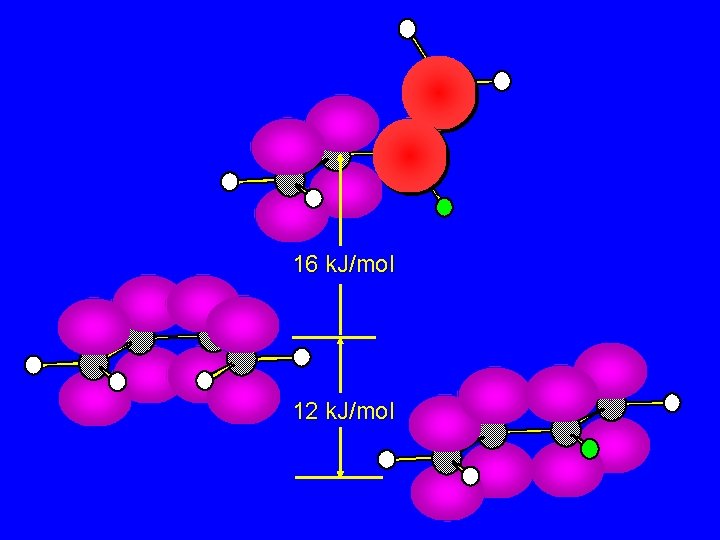
s-trans is more stable than s-cis Interconversion of conformations requires two p bonds to be at right angles to each other and prevents conjugation 12 k. J/mol


16 k. J/mol 12 k. J/mol

10. 8 Bonding in Allenes

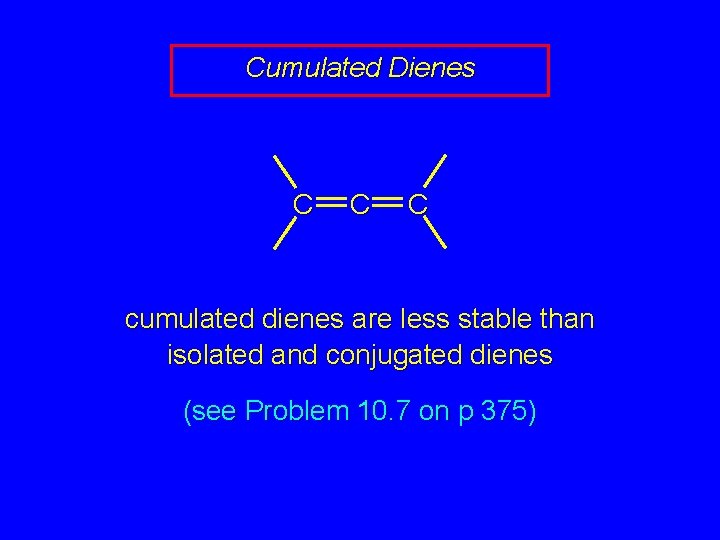
Cumulated Dienes C C C cumulated dienes are less stable than isolated and conjugated dienes (see Problem 10. 7 on p 375)

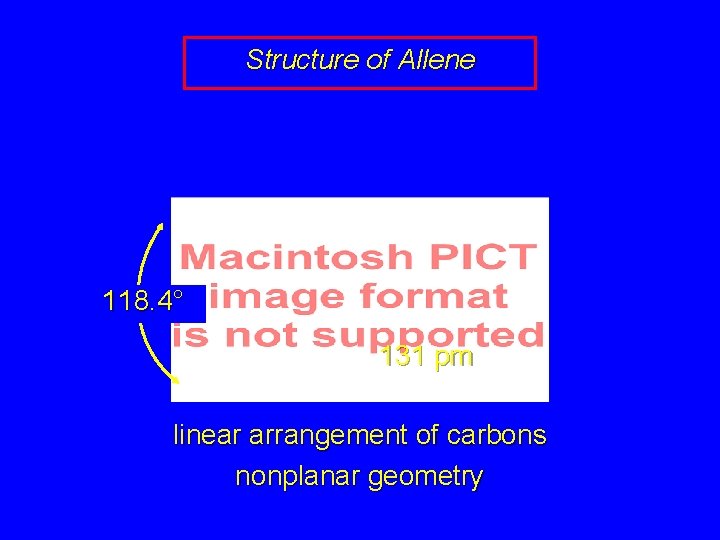
Structure of Allene 118. 4° 131 pm linear arrangement of carbons nonplanar geometry

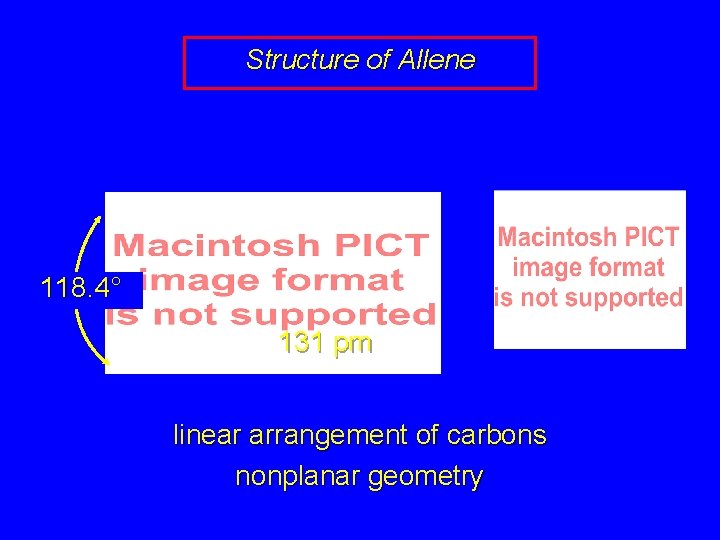
Structure of Allene 118. 4° 131 pm linear arrangement of carbons nonplanar geometry

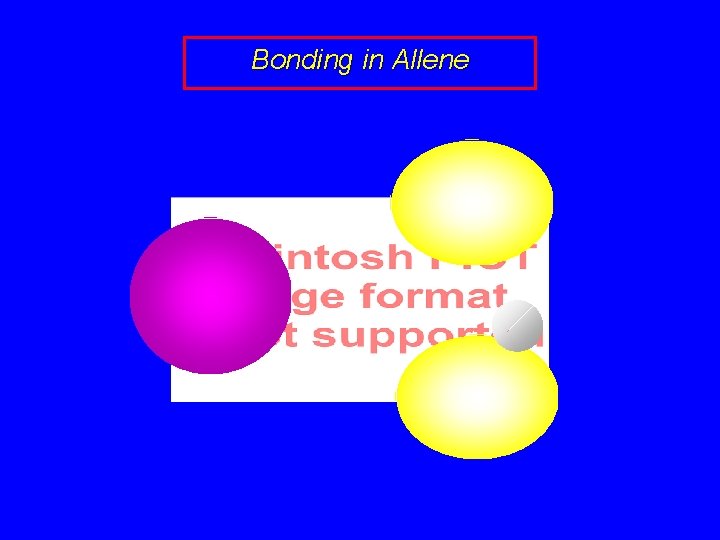
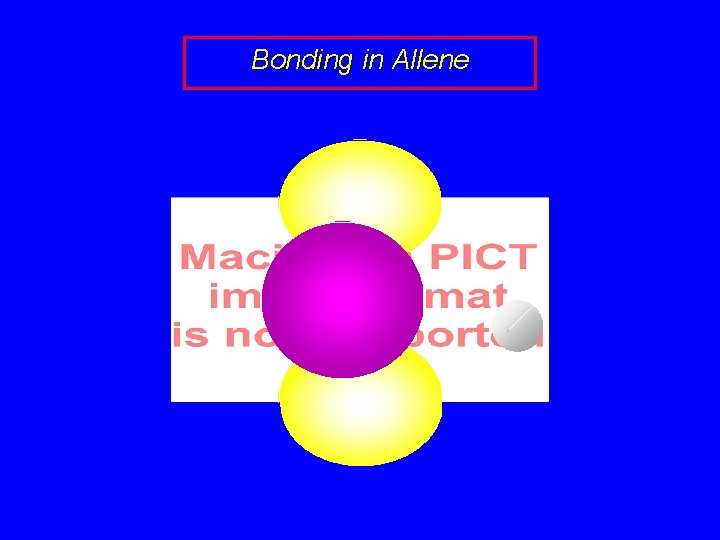
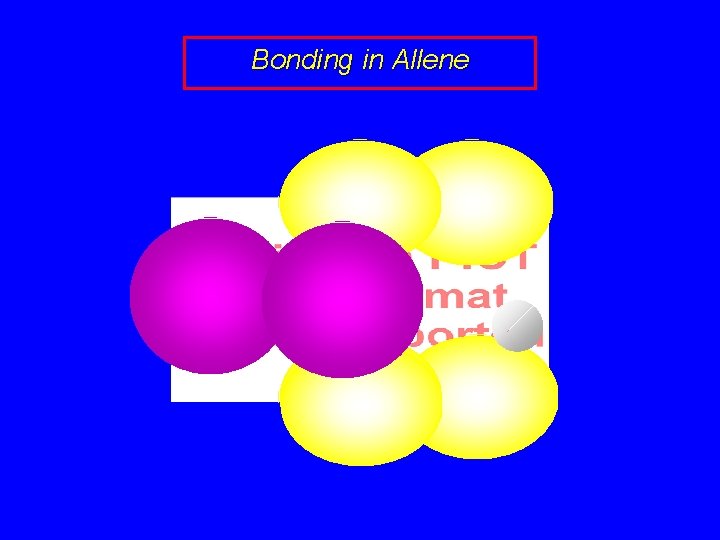
Bonding in Allene sp 2 sp sp 2

Bonding in Allene

Bonding in Allene

Bonding in Allene

Chiral Allenes of the type shown are chiral X A C C C Y B A π B; X π Y Have a stereogenic axis

Stereogenic Axis analogous to difference between: a screw with a right-hand thread and one with a left-hand thread a right-handed helix and a left-handed helix
 Cumulated diene
Cumulated diene Clases e subclasses
Clases e subclasses Pre ap classes vs regular classes
Pre ap classes vs regular classes Library of congress classification
Library of congress classification Teori dienes
Teori dienes Alat peraga geometri sma
Alat peraga geometri sma Effetto dienes
Effetto dienes Effetto dienes
Effetto dienes Conjugated dienes
Conjugated dienes Konstruktivitás
Konstruktivitás Bathochromic shift and hypsochromic shift
Bathochromic shift and hypsochromic shift Diene
Diene Bloques dienes
Bloques dienes Double bond extending conjugation
Double bond extending conjugation Uv spectra of dienes
Uv spectra of dienes Preparation of dienes
Preparation of dienes Follicular conjunctivitis
Follicular conjunctivitis Dienes blocks
Dienes blocks Eager learner examples
Eager learner examples Chronological tabulation is based on
Chronological tabulation is based on Traditional classification vs modern classification
Traditional classification vs modern classification Three classes of yeast breads
Three classes of yeast breads Worms and mollusks
Worms and mollusks Jmu gen ed planner
Jmu gen ed planner Ivc summer classes for high school students
Ivc summer classes for high school students Stream classes in c++
Stream classes in c++ Ancient china hierarchy pyramid
Ancient china hierarchy pyramid Social classes on the titanic
Social classes on the titanic Song dynasty social structure
Song dynasty social structure Federally protected classes
Federally protected classes Canterbury tales social classes chart
Canterbury tales social classes chart Aztec hierarchy pyramid
Aztec hierarchy pyramid