10 3 Percent Composition and Chemical Formulas Chapter


- Slides: 13

10. 3 Percent Composition and Chemical Formulas > Chapter 10 Chemical Quantities 10. 1 The Mole: A Measurement of Matter 10. 2 Mole-Mass and Mole-Volume Relationships 10. 3 Percent Composition and Chemical Formulas 1 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. .

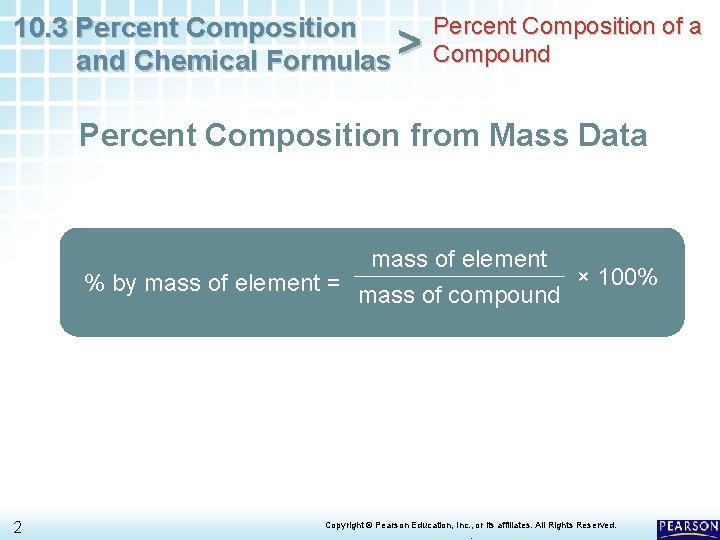
Percent Composition of a 10. 3 Percent Composition and Chemical Formulas > Compound Percent Composition from Mass Data mass of element % by mass of element = mass of compound × 100% 2 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. .

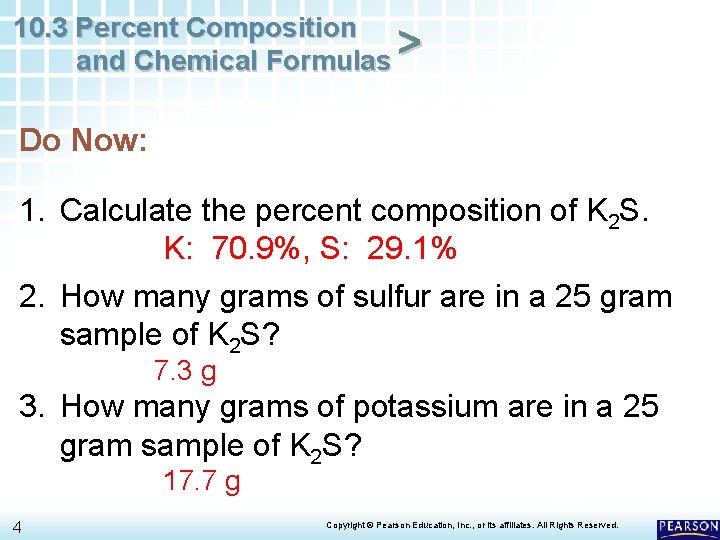
10. 3 Percent Composition and Chemical Formulas > Do Now: 1. Calculate the percent composition of K 2 S. 2. How many grams of sulfur are in a 25 gram sample of K 2 S? 3. How many grams of potassium are in a 25 gram sample of K 2 S? 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

10. 3 Percent Composition and Chemical Formulas > Do Now: 1. Calculate the percent composition of K 2 S. K: 70. 9%, S: 29. 1% 2. How many grams of sulfur are in a 25 gram sample of K 2 S? 7. 3 g 3. How many grams of potassium are in a 25 gram sample of K 2 S? 17. 7 g 4 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

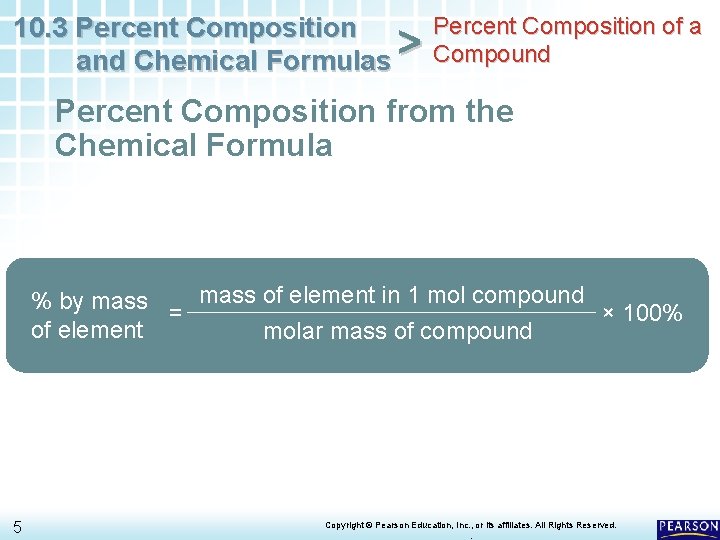
Percent Composition of a 10. 3 Percent Composition and Chemical Formulas > Compound Percent Composition from the Chemical Formula % by mass = mass of element in 1 mol compound × 100% of element molar mass of compound 5 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. .

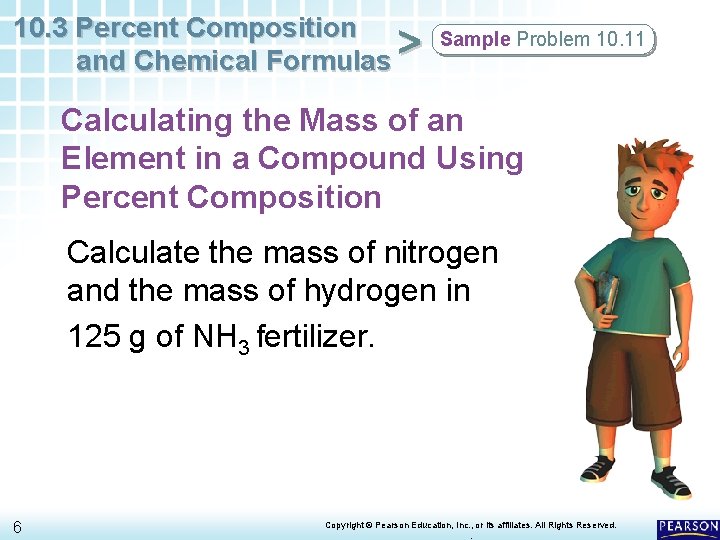
10. 3 Percent Composition and Chemical Formulas > Sample Problem 10. 11 Calculating the Mass of an Element in a Compound Using Percent Composition Calculate the mass of nitrogen and the mass of hydrogen in 125 g of NH 3 fertilizer. 6 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. .

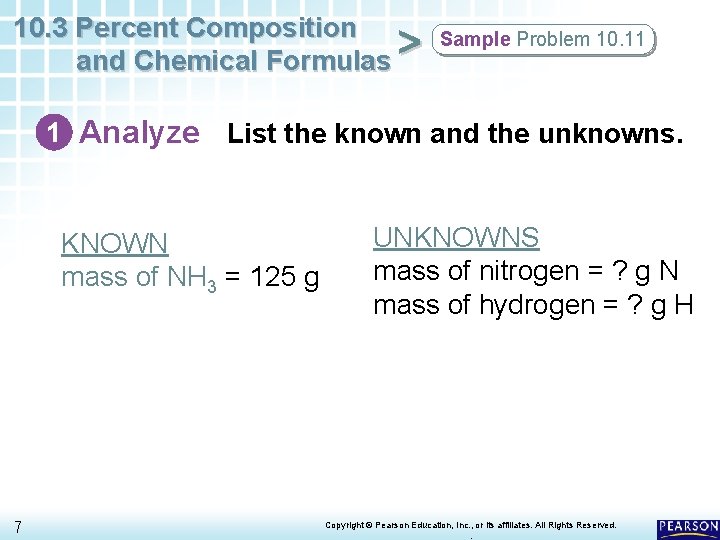
10. 3 Percent Composition and Chemical Formulas > Sample Problem 10. 11 1 Analyze List the known and the unknowns. KNOWN mass of NH 3 = 125 g 7 UNKNOWNS mass of nitrogen = ? g N mass of hydrogen = ? g H Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. .

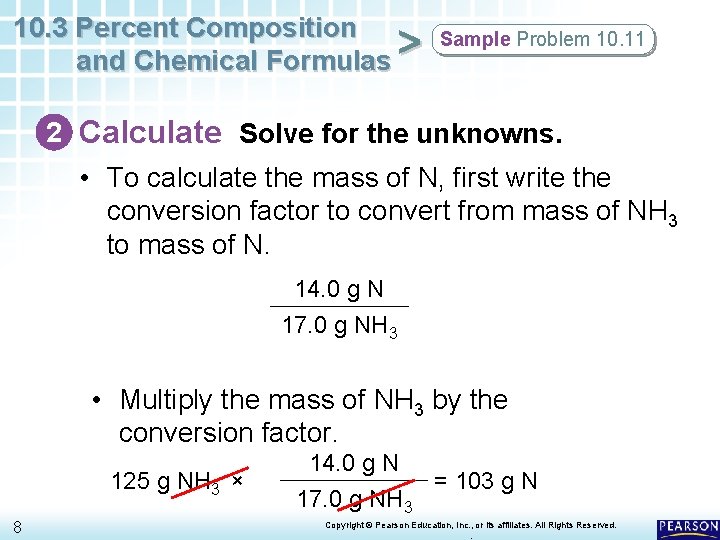
10. 3 Percent Composition and Chemical Formulas > Sample Problem 10. 11 2 Calculate Solve for the unknowns. • To calculate the mass of N, first write the conversion factor to convert from mass of NH 3 to mass of N. 14. 0 g N 17. 0 g NH 3 • Multiply the mass of NH 3 by the conversion factor. 125 g NH 3 × 8 14. 0 g N = 103 g N 17. 0 g NH 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. .

10. 3 Percent Composition and Chemical Formulas > Sample Problem 10. 11 2 Calculate Solve for the unknowns. • To calculate the mass of H, first write the conversion factor to convert from mass of NH 3 to mass of H. 3 g. H 17 g NH 3 • Multiply the mass of NH 3 by the conversion factor. 125 g NH 3 × 9 3 g. H 17 g NH 3 = 22 g H Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. .

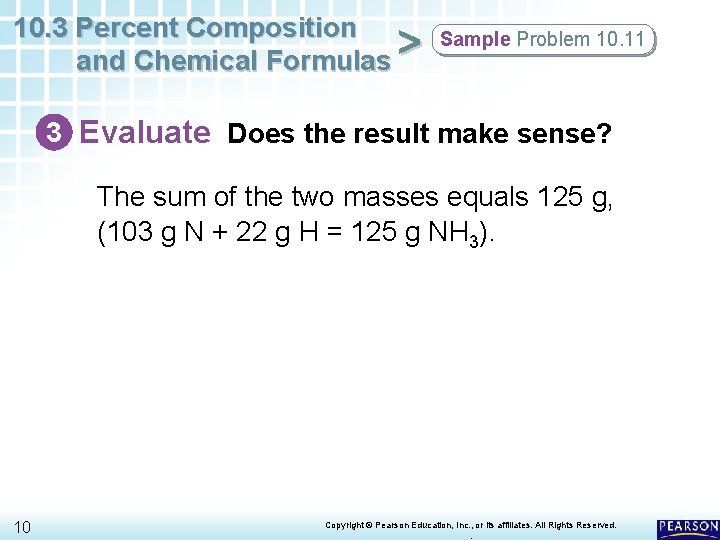
10. 3 Percent Composition and Chemical Formulas > Sample Problem 10. 11 3 Evaluate Does the result make sense? The sum of the two masses equals 125 g, (103 g N + 22 g H = 125 g NH 3). 10 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. .

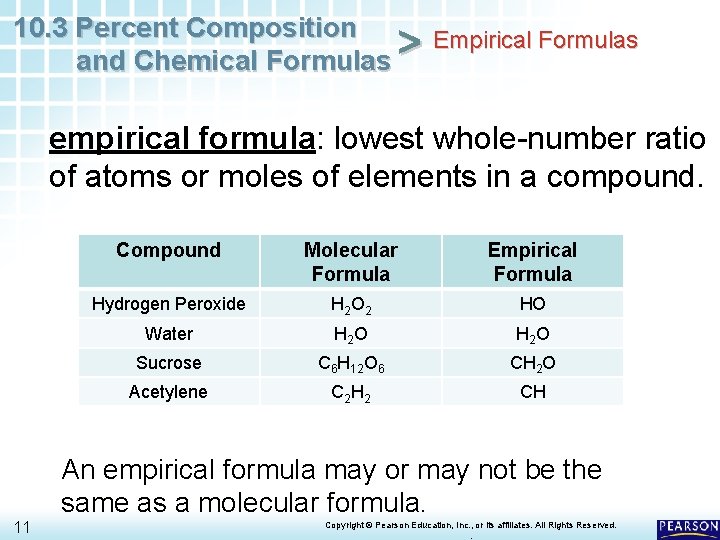
10. 3 Percent Composition Empirical Formulas > and Chemical Formulas empirical formula: lowest whole-number ratio of atoms or moles of elements in a compound. 11 Compound Molecular Formula Empirical Formula Hydrogen Peroxide H 2 O 2 HO Water H 2 O Sucrose C 6 H 12 O 6 CH 2 O Acetylene C 2 H 2 CH An empirical formula may or may not be the same as a molecular formula. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. .

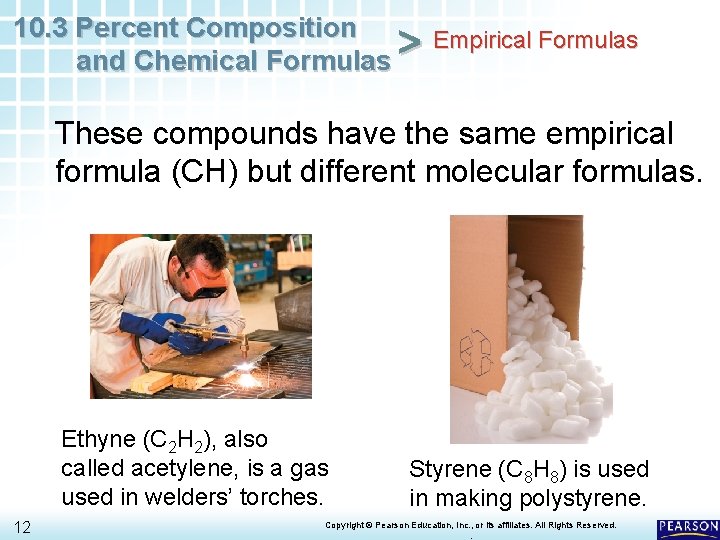
10. 3 Percent Composition Empirical Formulas > and Chemical Formulas These compounds have the same empirical formula (CH) but different molecular formulas. Ethyne (C 2 H 2), also called acetylene, is a gas used in welders’ torches. 12 Styrene (C 8 H 8) is used in making polystyrene. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. .

10. 3 Percent Composition and Chemical Formulas > END OF 10. 3 13 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. .