10 3 Equilibrium Constants At equilibrium the number

10. 3 Equilibrium Constants At equilibrium, the number of people riding up the lift and the number of people skiing down the slope are constant. Learning Goal Calculate the equilibrium constant for a reversible reaction given the concentrations of reactants and products at equilibrium. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
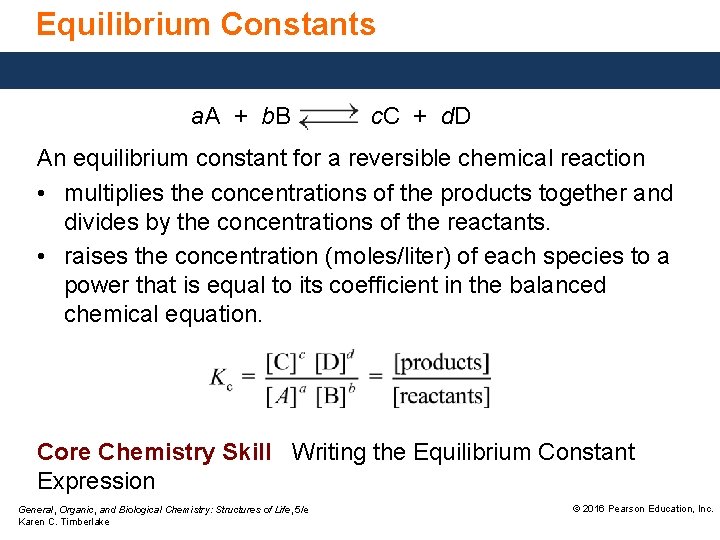
Equilibrium Constants a. A + b. B c. C + d. D An equilibrium constant for a reversible chemical reaction • multiplies the concentrations of the products together and divides by the concentrations of the reactants. • raises the concentration (moles/liter) of each species to a power that is equal to its coefficient in the balanced chemical equation. Core Chemistry Skill Writing the Equilibrium Constant Expression General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
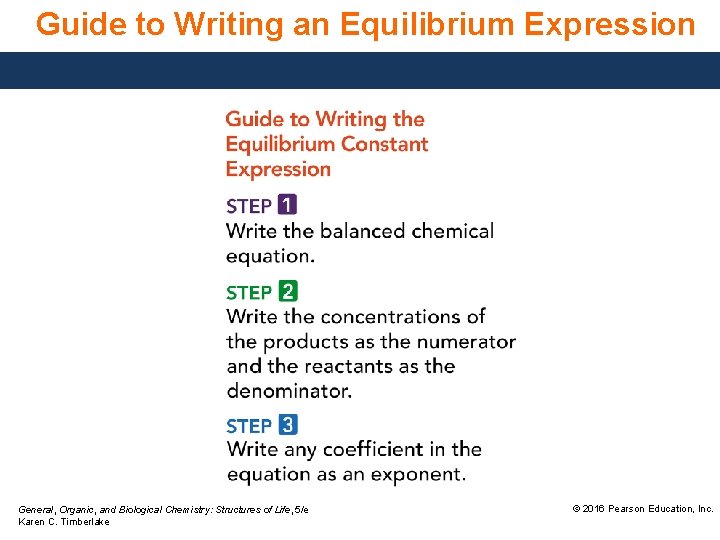
Guide to Writing an Equilibrium Expression General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
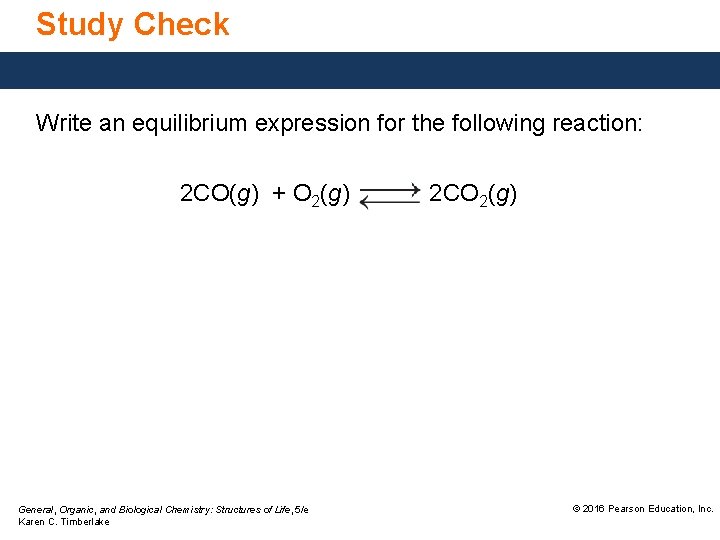
Study Check Write an equilibrium expression for the following reaction: 2 CO(g) + O 2(g) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake 2 CO 2(g) © 2016 Pearson Education, Inc.
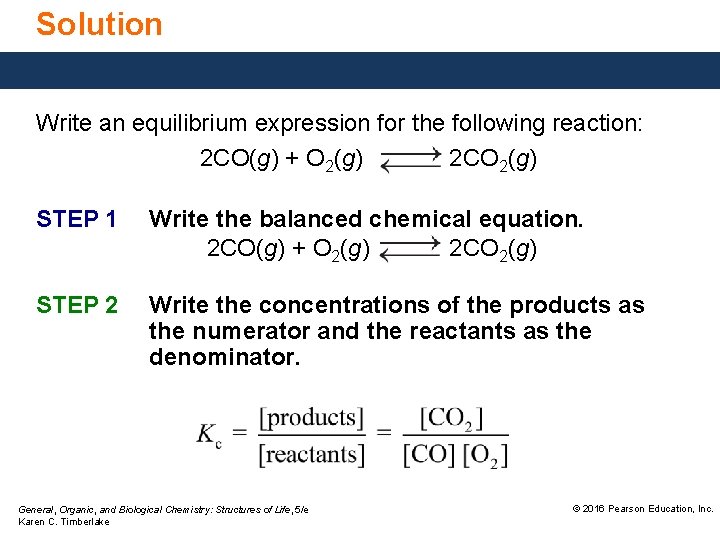
Solution Write an equilibrium expression for the following reaction: 2 CO(g) + O 2(g) 2 CO 2(g) STEP 1 Write the balanced chemical equation. 2 CO(g) + O 2(g) 2 CO 2(g) STEP 2 Write the concentrations of the products as the numerator and the reactants as the denominator. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
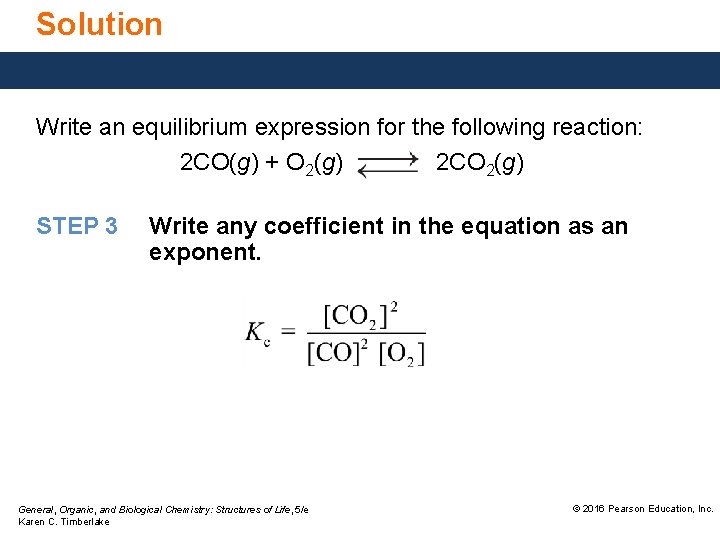
Solution Write an equilibrium expression for the following reaction: 2 CO(g) + O 2(g) 2 CO 2(g) STEP 3 Write any coefficient in the equation as an exponent. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
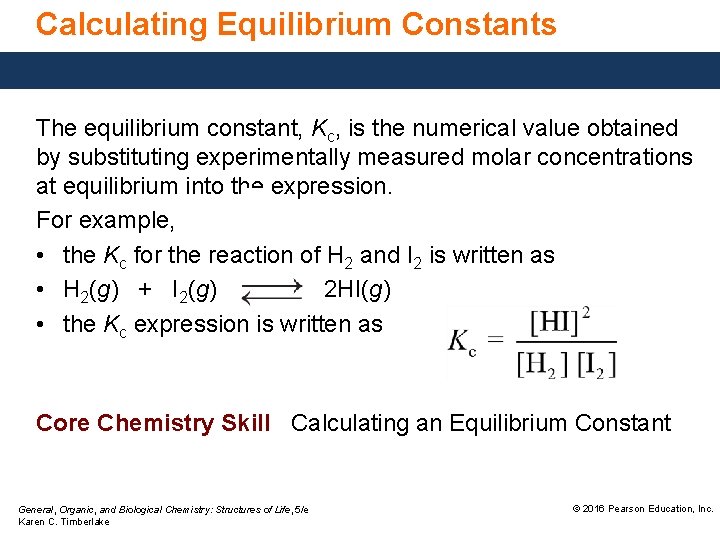
Calculating Equilibrium Constants The equilibrium constant, Kc, is the numerical value obtained by substituting experimentally measured molar concentrations at equilibrium into the expression. For example, • the Kc for the reaction of H 2 and I 2 is written as • H 2(g) + I 2(g) 2 HI(g) • the Kc expression is written as Core Chemistry Skill Calculating an Equilibrium Constant General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
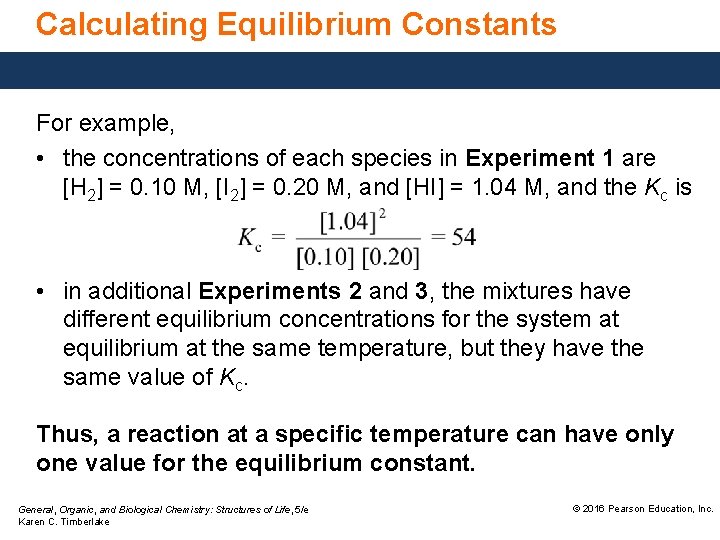
Calculating Equilibrium Constants For example, • the concentrations of each species in Experiment 1 are [H 2] = 0. 10 M, [I 2] = 0. 20 M, and [HI] = 1. 04 M, and the Kc is • in additional Experiments 2 and 3, the mixtures have different equilibrium concentrations for the system at equilibrium at the same temperature, but they have the same value of Kc. Thus, a reaction at a specific temperature can have only one value for the equilibrium constant. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
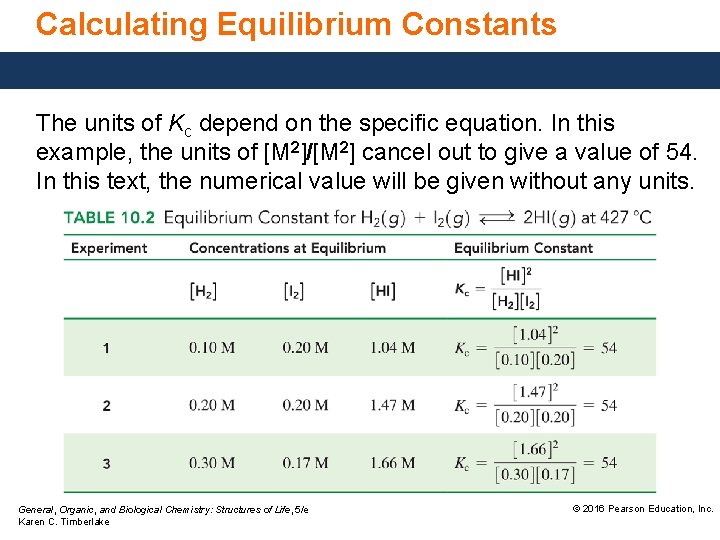
Calculating Equilibrium Constants The units of Kc depend on the specific equation. In this example, the units of [M 2]/[M 2] cancel out to give a value of 54. In this text, the numerical value will be given without any units. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
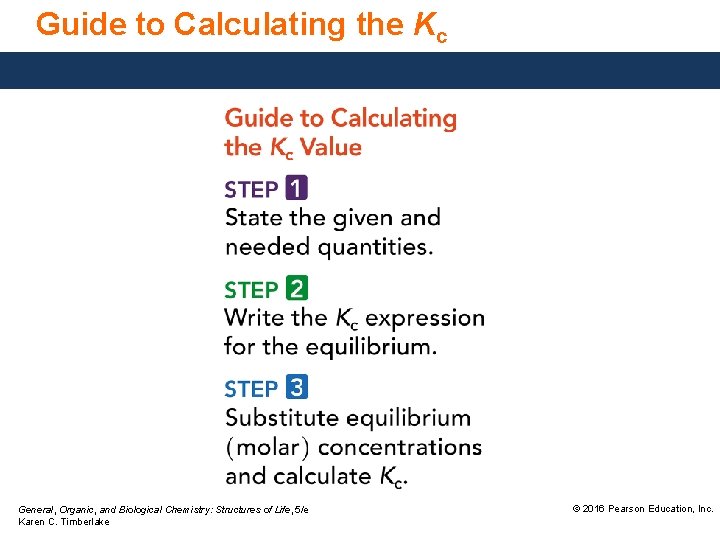
Guide to Calculating the Kc General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
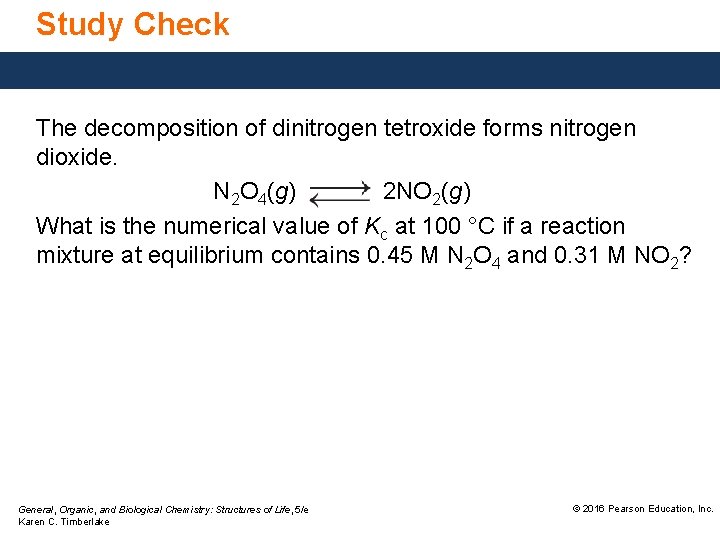
Study Check The decomposition of dinitrogen tetroxide forms nitrogen dioxide. N 2 O 4(g) 2 NO 2(g) What is the numerical value of Kc at 100 °C if a reaction mixture at equilibrium contains 0. 45 M N 2 O 4 and 0. 31 M NO 2? General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
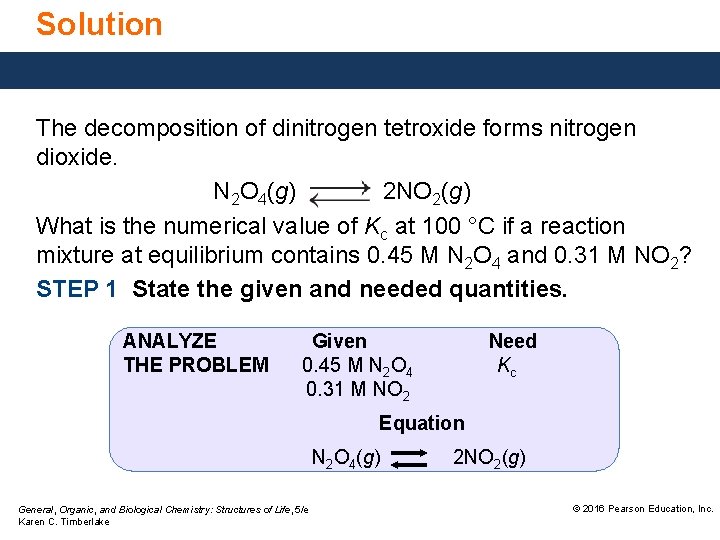
Solution The decomposition of dinitrogen tetroxide forms nitrogen dioxide. N 2 O 4(g) 2 NO 2(g) What is the numerical value of Kc at 100 °C if a reaction mixture at equilibrium contains 0. 45 M N 2 O 4 and 0. 31 M NO 2? STEP 1 State the given and needed quantities. ANALYZE THE PROBLEM Given 0. 45 M N 2 O 4 0. 31 M NO 2 Need Kc Equation N 2 O 4(g) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake 2 NO 2(g) © 2016 Pearson Education, Inc.
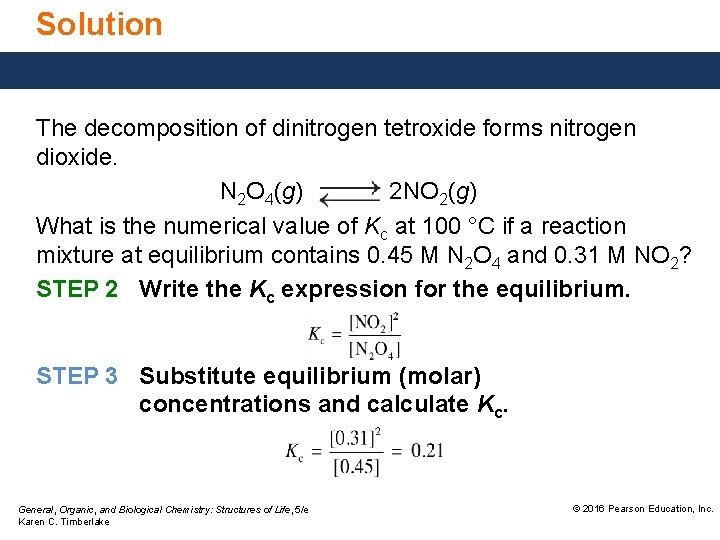
Solution The decomposition of dinitrogen tetroxide forms nitrogen dioxide. N 2 O 4(g) 2 NO 2(g) What is the numerical value of Kc at 100 °C if a reaction mixture at equilibrium contains 0. 45 M N 2 O 4 and 0. 31 M NO 2? STEP 2 Write the Kc expression for the equilibrium. STEP 3 Substitute equilibrium (molar) concentrations and calculate Kc. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
Study Check Given the following chemical reaction, H 2(g) + I 2(g) 2 HI(g) what is the numerical value of Kc at 443 °C if the equilibrium concentrations are as follows? [H 2] = 1. 2 M [I 2] = 1. 2 M [HI] = 0. 35 M General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
Solution Given the following chemical reaction, H 2(g) + I 2(g) 2 HI(g) what is the numerical value of Kc at 443 °C if the equilibrium concentrations are as follows? [H 2] = 1. 2 M [I 2] = 1. 2 M [HI] = 0. 35 M STEP 1 State the given and needed quantities. ANALYZE THE PROBLEM Given 1. 2 M H 2, 1. 2 M I 2, 0. 35 M HI Need Kc Equation H 2(g) + I 2(g) 2 HI(g) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
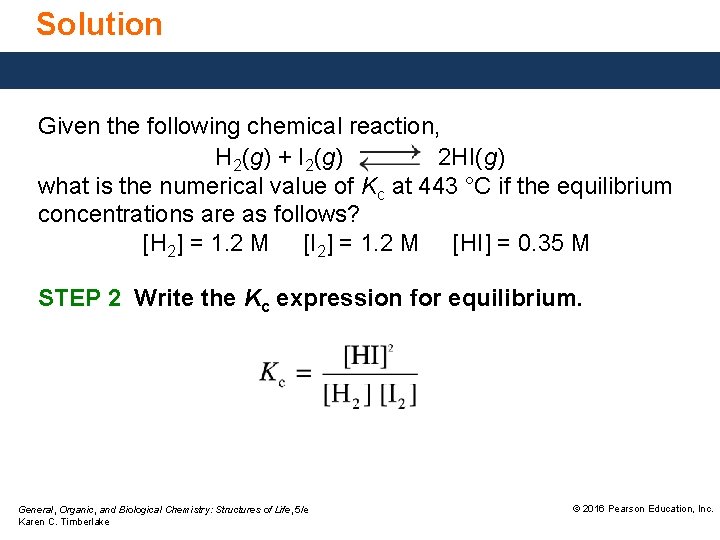
Solution Given the following chemical reaction, H 2(g) + I 2(g) 2 HI(g) what is the numerical value of Kc at 443 °C if the equilibrium concentrations are as follows? [H 2] = 1. 2 M [I 2] = 1. 2 M [HI] = 0. 35 M STEP 2 Write the Kc expression for equilibrium. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
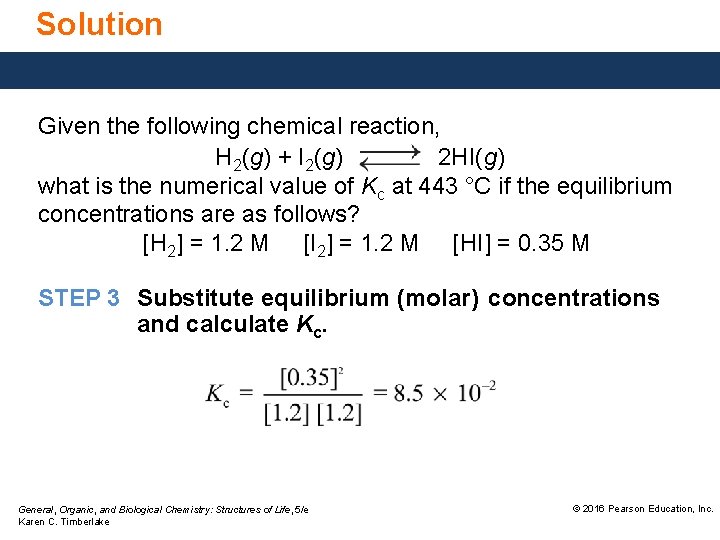
Solution Given the following chemical reaction, H 2(g) + I 2(g) 2 HI(g) what is the numerical value of Kc at 443 °C if the equilibrium concentrations are as follows? [H 2] = 1. 2 M [I 2] = 1. 2 M [HI] = 0. 35 M STEP 3 Substitute equilibrium (molar) concentrations and calculate Kc. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
- Slides: 17