10 3 AcidBase Reactions Acids And Bases Have
10. 3 Acid-Base Reactions
“Acids And Bases Have Two Different Faces” Coolest Song Ever!

Neutralization Reaction • Neutralization Rxn. A double displacement reaction in which an acid and a base combine to form water and a salt. • Salt. An ionic compound that is made from the anion from an acid and a cation from a base.
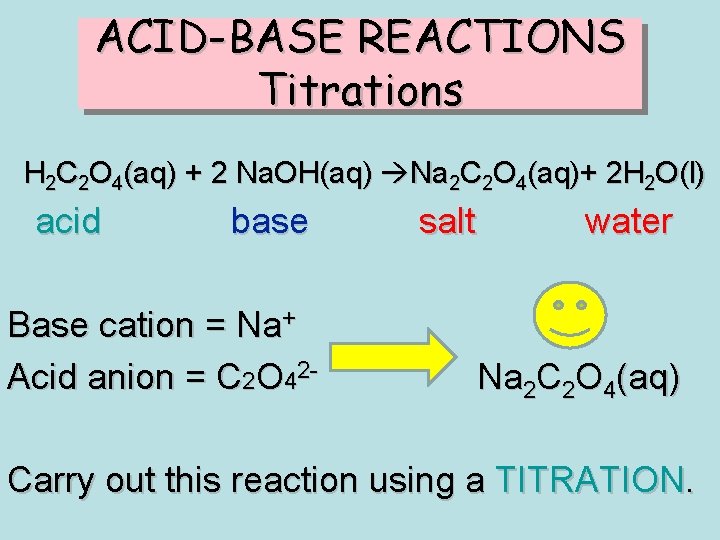
ACID-BASE REACTIONS Titrations H 2 C 2 O 4(aq) + 2 Na. OH(aq) Na 2 C 2 O 4(aq)+ 2 H 2 O(l) acid base Base cation = Na+ Acid anion = C 2 O 42 - salt water Na 2 C 2 O 4(aq) Carry out this reaction using a TITRATION.

Adding Acid and Bases • When a strong acid reacts with a strong base in the same mole ratio from the balanced --- a neutral aqueous solution of a salt and water is formed. • Why is this important? p. H =7
What is an Acid-Base Titration? • Acid-base titrationis a chemical analysis involving the addition of a known concentration of titrant to a known volume but unknown concentration of sample. • Chemical Indicatoris used to determine the end point of the reaction.
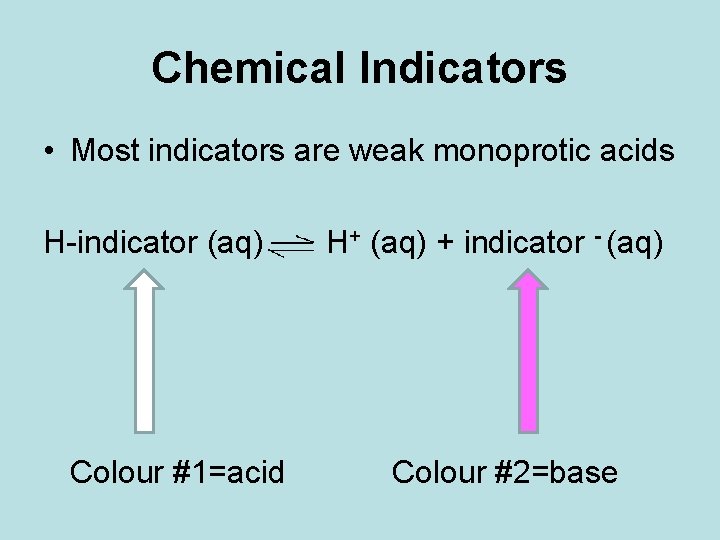
Chemical Indicators • Most indicators are weak monoprotic acids H-indicator (aq) Colour #1=acid H+ (aq) + indicator - (aq) Colour #2=base

• Titration Between a Strong Acid and Strong Base • The equivalence point will occur at a p. H of 7. • Want an indicator that will change around 7. • Titration Between a Weak Acid and Strong Base • The equivalence point will occur at a p. H above 7. • Want an indicator that will change above 7. • Titration Between a Strong Acid and Weak Base • The equivalence point will occur at a p. H below 7. • Want an indicator that will change below 7.
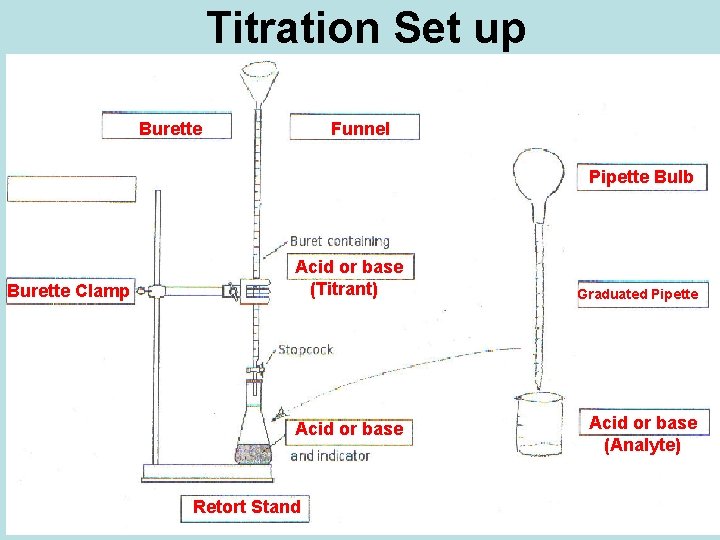
Titration Set up Burette Funnel Pipette Bulb Burette Clamp Acid or base (Titrant) Acid or base Retort Stand Graduated Pipette Acid or base (Analyte)
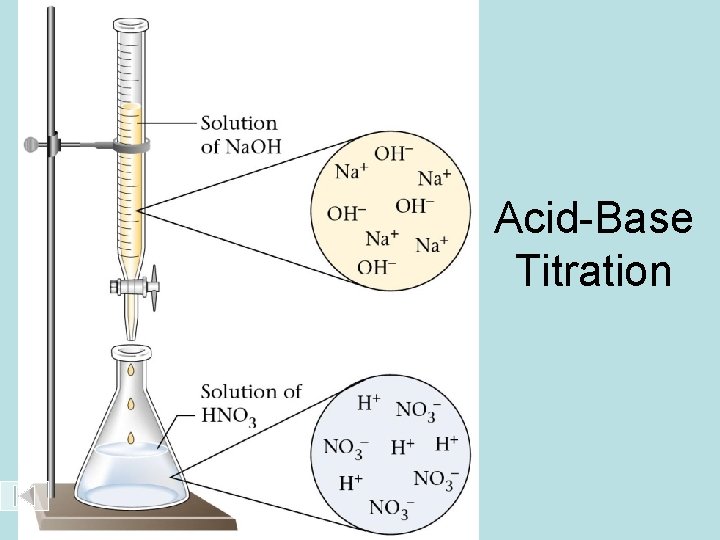
Acid-Base Titration

Setup for titrating an acid with a base
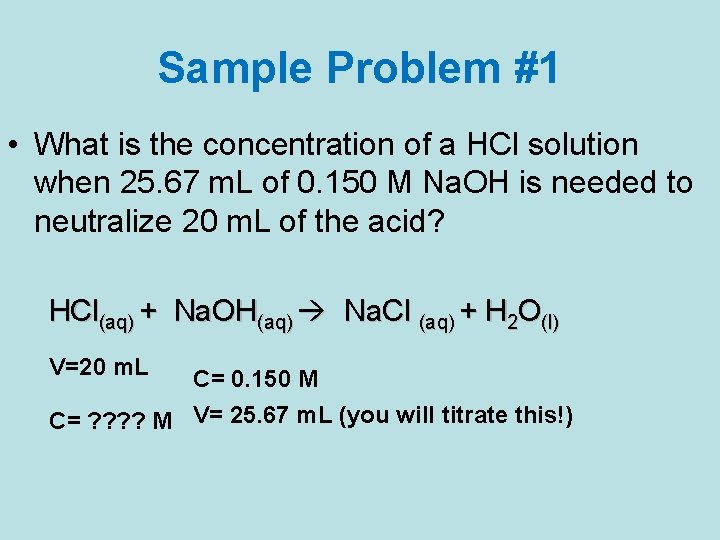
Sample Problem #1 • What is the concentration of a HCl solution when 25. 67 m. L of 0. 150 M Na. OH is needed to neutralize 20 m. L of the acid? HCl(aq) + Na. OH(aq) Na. Cl (aq) + H 2 O(l) V=20 m. L C= 0. 150 M C= ? ? M V= 25. 67 m. L (you will titrate this!)
Solve the problem • 1 st write the equation for the reaction: HCl(aq) + Na. OH(aq) → Na. Cl(aq) + H 2 O(l) • 2 nd solve for the amount of moles of the titrant used. n. Na. OH mol = Cx. V= 0. 150 mol/L x 0. 02567 L = 3. 85 x 10 -3 mol Na. OH Volume found in titration experiment • 3 rd using stoichiometry, solve for the concentration of HCl , knowing it is a 1: 1 mole ratio C= n = 3. 85 x 10 -3 mol= 0. 192 M V 0. 02000 L
Sample Problem #2 • What volume of 0. 400 mol/L of Mg(OH)2 is needed to react completely with 43. 5 m. L of 0. 781 mol/L of HCl? Mg(OH)2 + 2 HCl 2 H 2 O (l) + Mg. Cl 2 (aq) C=0. 400 mol/L C=0. 781 mol/L V= ? V= 43. 5 m. L 1. Determine number of moles of HCl: n. HCl= Cx. V = (0. 781 mol/L) (0. 0435 L) =0. 03315 mol 2. Mole Ratio! Mg(OH)2 : HCl 1: 2 X: 0. 03315 mol / 2 mol n Mg(OH)2 = 0. 0166 mol
3. FIX THIS SLIDE! You now have the number of moles of Mg(OH)2 You can now find the volume of the analyte. VHCl= n = 0. 0166 mol = 0. 0414 L C 0. 400 mol/L 0. 0414 L x 1000 ml = 41. 4 m. L 1 L
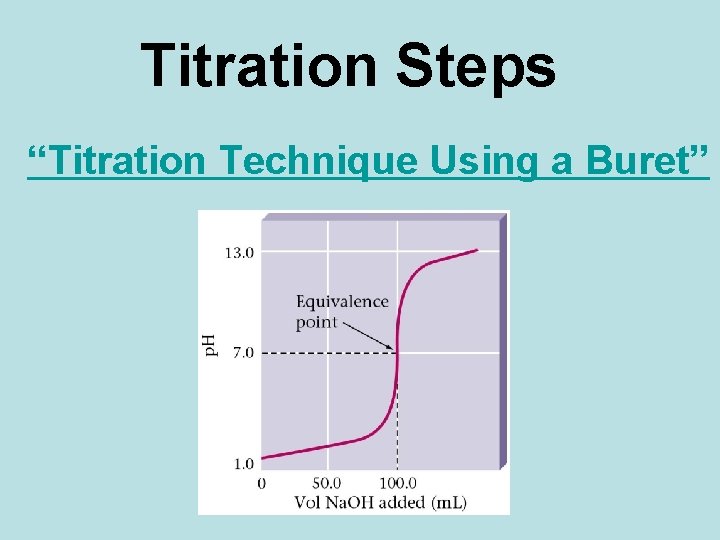
Titration Steps “Titration Technique Using a Buret”

• Step 1: The Na. OH, the titrant, is placed in the buret. The titrant is the solution of known concentration that is added from the buret into the flask with the unknown called the analyte.
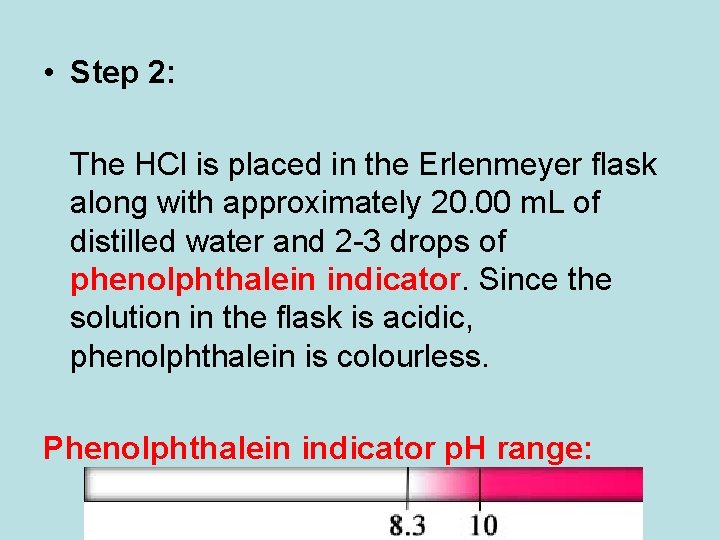
• Step 2: The HCl is placed in the Erlenmeyer flask along with approximately 20. 00 m. L of distilled water and 2 -3 drops of phenolphthalein indicator. Since the solution in the flask is acidic, phenolphthalein is colourless. Phenolphthalein indicator p. H range:

• Step 3: Na. OH is added to the HCl in the flask. When the Na. OH comes in contact with the solution in the flask, it turns pink and then the pink colour quickly disappears. This is because the OH- from the Na. OH interact with the phenolphthalein to change the phenolphthalein from colourless to pink.

• The solution becomes clear again as the hydronium ions from the hydrochloric acid neutralize the added hydroxide ions. As more Na. OH is added, it takes longer for the pink colour to disappear. • As it starts taking longer for the pink colour to disappear, the sodium hydroxide is added a drop at a time.

• The equivalence point of the titration is reached when equal numbers of moles of hydronium and hydroxide ions have been reacted. • When this happens in this titration, the p. H of the solution in the flask is 7. 0 and the phenolphthalein indicator is colourless. • This would be a good time to stop, however the indicator is still colourless, so must keep going.
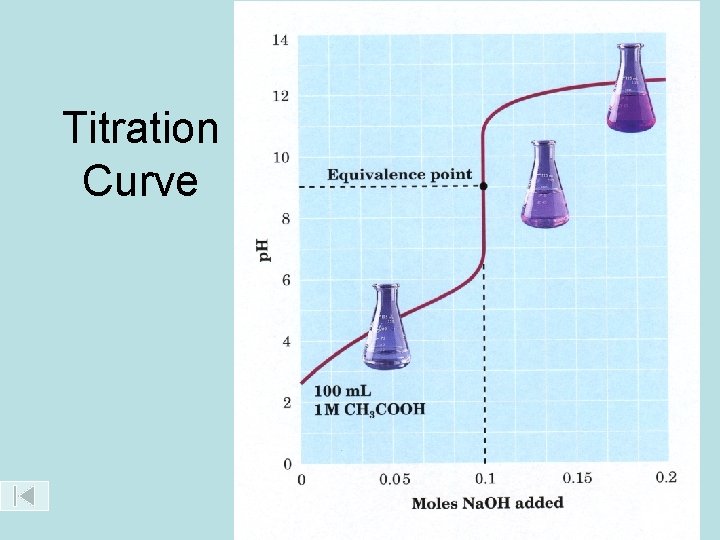
Titration Curve

Step 4: • Add as little excess Na. OH as possible. We want to add a single drop of Na. OH to the colourless solution in the flask and have the solution in the flask turn pink and stay pink while the contents of the flask are swirled. • This permanent colour change in the indicator is known as the endpoint of the titration and the titration is over.

Homework SECTION 10. 3 PRACTICE PROBLEMS • Page 398, #12, 13.
- Slides: 24