10 2 Neutralization and AcidBase Titrations Learning Goals

10. 2 Neutralization and Acid-Base Titrations Learning Goals … … use stoichiometry to calculate volumes and concentrations in a neutralization reaction … determine the p. H of a solution with an excess reactant
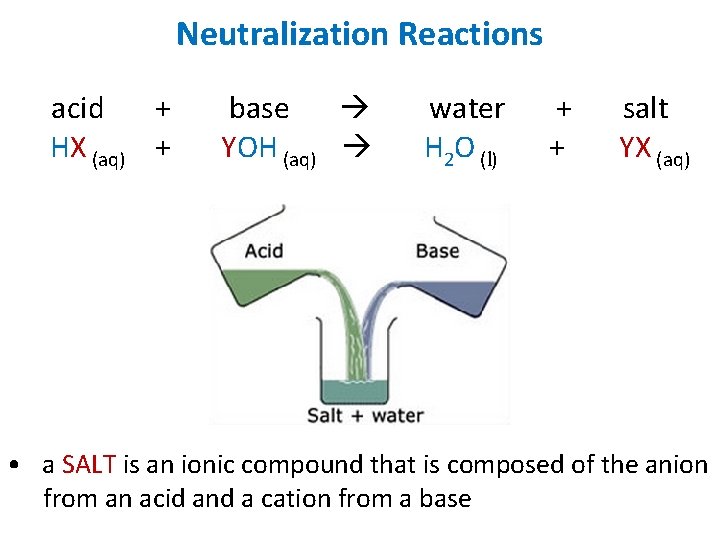
Neutralization Reactions acid + HX (aq) + base YOH (aq) water H 2 O (l) + + salt YX (aq) • a SALT is an ionic compound that is composed of the anion from an acid and a cation from a base
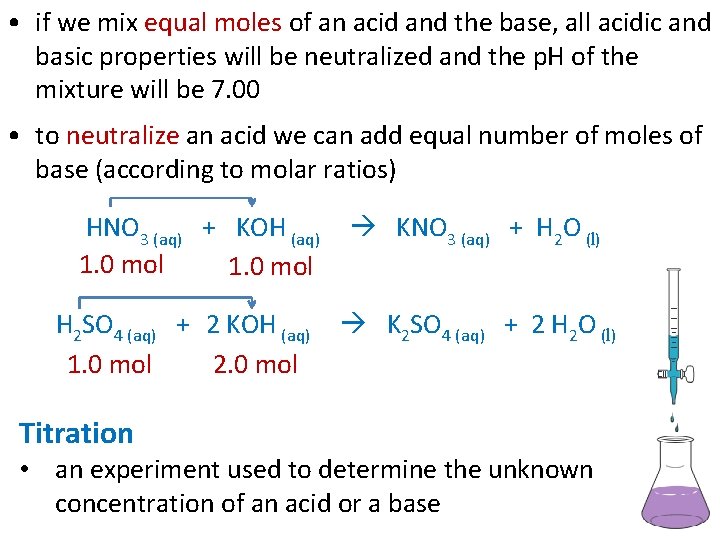
• if we mix equal moles of an acid and the base, all acidic and basic properties will be neutralized and the p. H of the mixture will be 7. 00 • to neutralize an acid we can add equal number of moles of base (according to molar ratios) HNO 3 (aq) + KOH (aq) 1. 0 mol H 2 SO 4 (aq) + 2 KOH (aq) 1. 0 mol 2. 0 mol Titration KNO 3 (aq) + H 2 O (l) K 2 SO 4 (aq) + 2 H 2 O (l) • an experiment used to determine the unknown concentration of an acid or a base
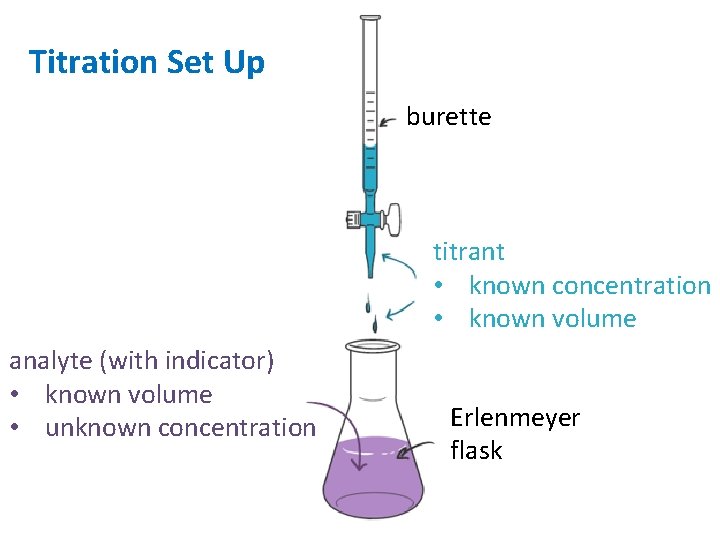
Titration Set Up burette titrant • known concentration • known volume analyte (with indicator) • known volume • unknown concentration Erlenmeyer flask
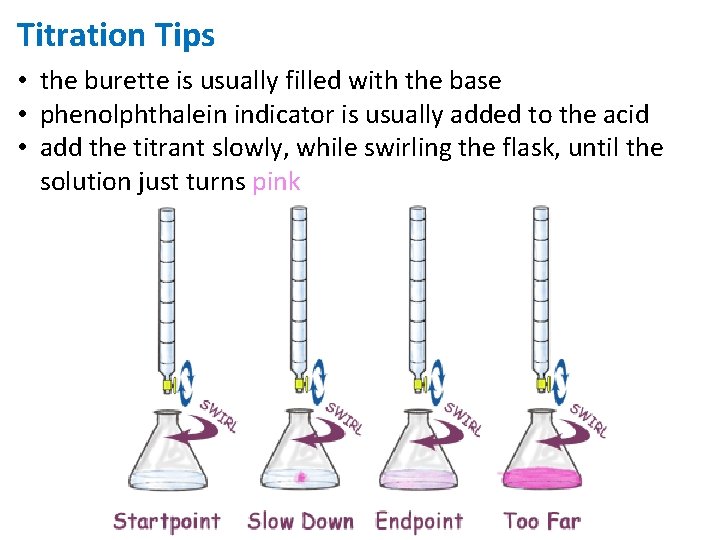
Titration Tips • the burette is usually filled with the base • phenolphthalein indicator is usually added to the acid • add the titrant slowly, while swirling the flask, until the solution just turns pink
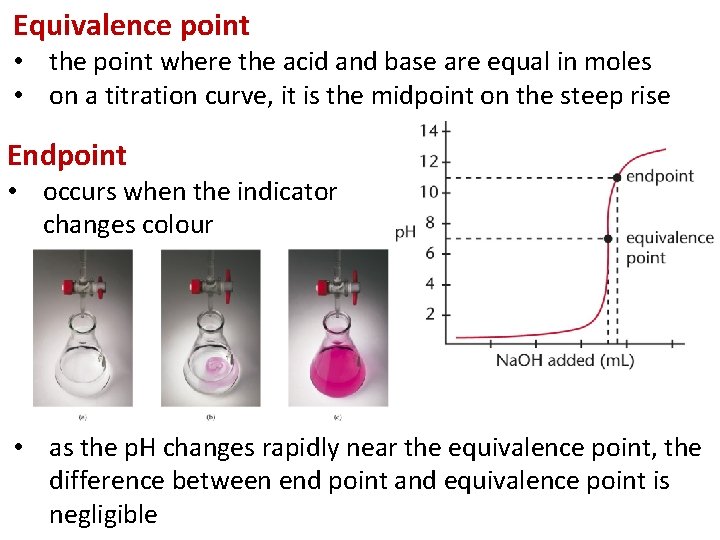
Equivalence point • the point where the acid and base are equal in moles • on a titration curve, it is the midpoint on the steep rise Endpoint • occurs when the indicator changes colour • as the p. H changes rapidly near the equivalence point, the difference between end point and equivalence point is negligible
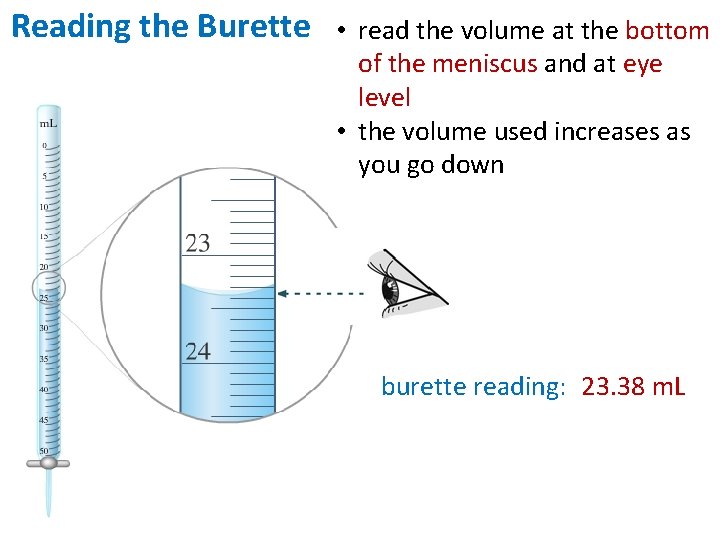
Reading the Burette • read the volume at the bottom of the meniscus and at eye level • the volume used increases as you go down burette reading: 23. 38 m. L
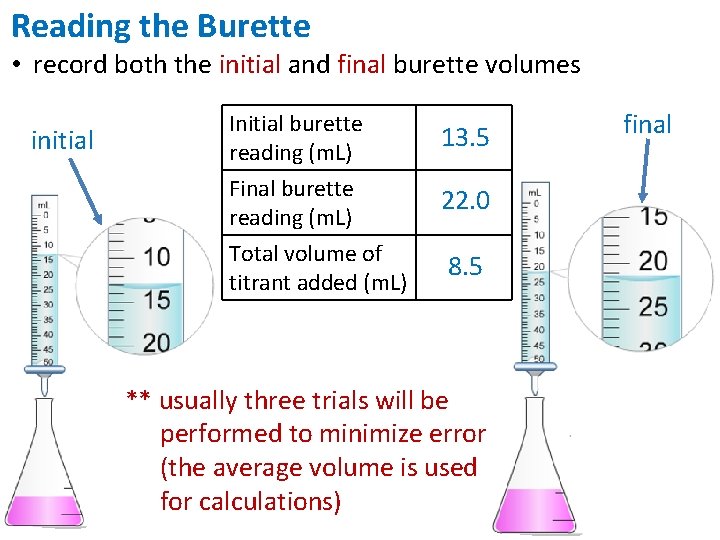
Reading the Burette • record both the initial and final burette volumes initial Initial burette reading (m. L) Final burette reading (m. L) 13. 5 Total volume of titrant added (m. L) 8. 5 22. 0 ** usually three trials will be performed to minimize error (the average volume is used for calculations) final
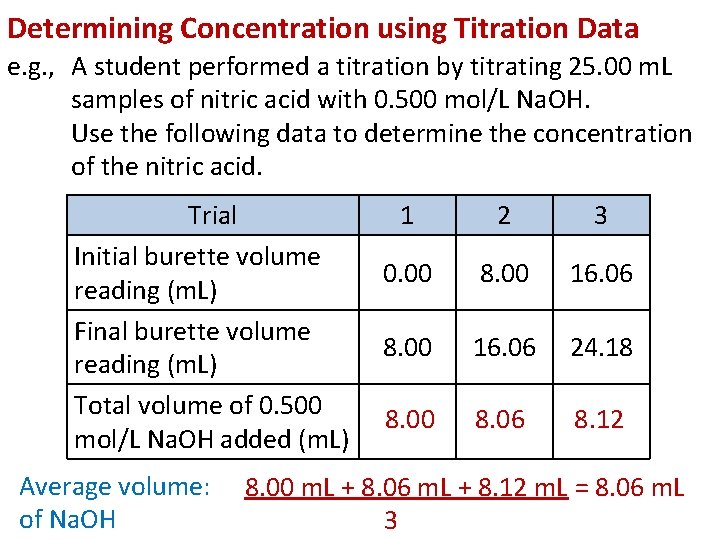
Determining Concentration using Titration Data e. g. , A student performed a titration by titrating 25. 00 m. L samples of nitric acid with 0. 500 mol/L Na. OH. Use the following data to determine the concentration of the nitric acid. Trial Initial burette volume reading (m. L) Final burette volume reading (m. L) Total volume of 0. 500 mol/L Na. OH added (m. L) Average volume: of Na. OH 1 2 3 0. 00 8. 00 16. 06 24. 18 8. 00 8. 06 8. 12 8. 00 m. L + 8. 06 m. L + 8. 12 m. L = 8. 06 m. L 3
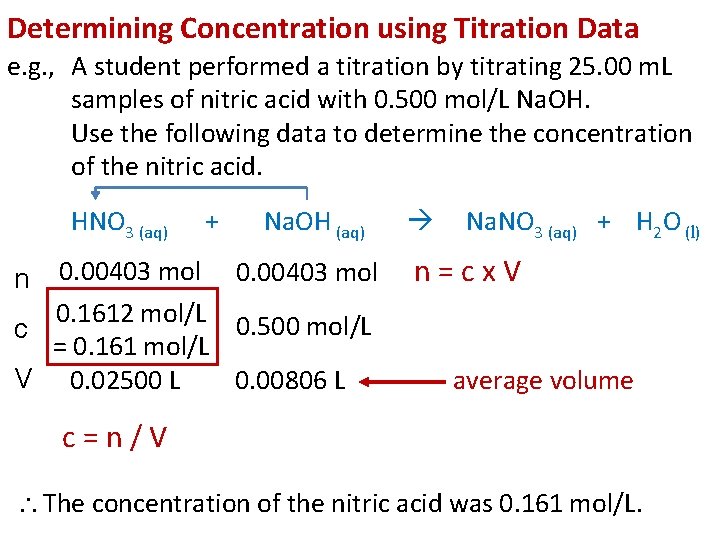
Determining Concentration using Titration Data e. g. , A student performed a titration by titrating 25. 00 m. L samples of nitric acid with 0. 500 mol/L Na. OH. Use the following data to determine the concentration of the nitric acid. HNO 3 (aq) + Na. OH (aq) 0. 00403 mol 0. 1612 mol/L 0. 500 mol/L c = 0. 161 mol/L V 0. 02500 L 0. 00806 L n Na. NO 3 (aq) + H 2 O (l) n=cx. V average volume c=n/V The concentration of the nitric acid was 0. 161 mol/L.
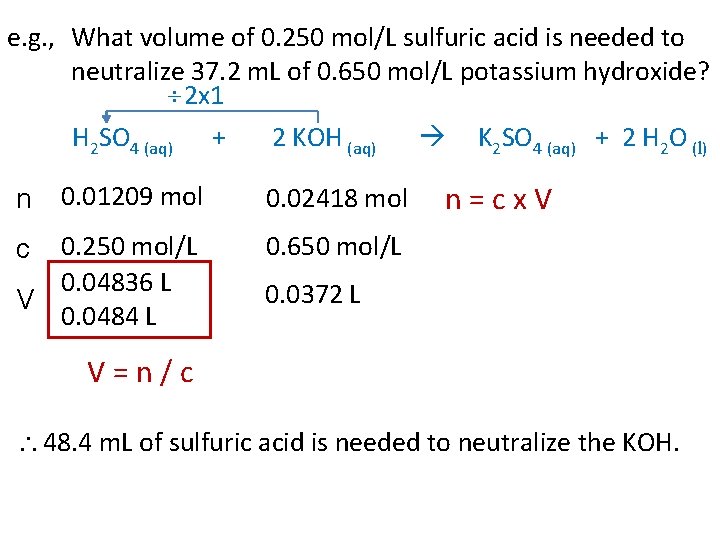
e. g. , What volume of 0. 250 mol/L sulfuric acid is needed to neutralize 37. 2 m. L of 0. 650 mol/L potassium hydroxide? 2 x 1 H 2 SO 4 (aq) + 2 KOH (aq) K 2 SO 4 (aq) + 2 H 2 O (l) 0. 01209 mol 0. 02418 mol 0. 250 mol/L 0. 04836 L V 0. 0484 L 0. 650 mol/L n c n=cx. V 0. 0372 L V=n/c 48. 4 m. L of sulfuric acid is needed to neutralize the KOH.
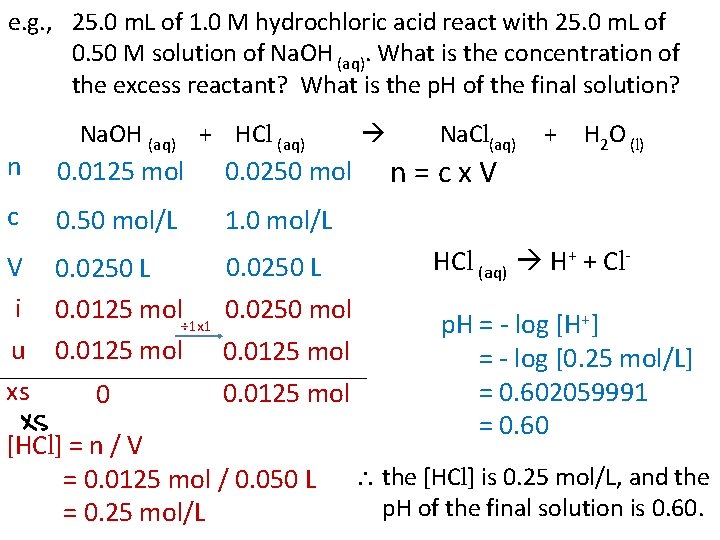
e. g. , 25. 0 m. L of 1. 0 M hydrochloric acid react with 25. 0 m. L of 0. 50 M solution of Na. OH (aq). What is the concentration of the excess reactant? What is the p. H of the final solution? Na. OH (aq) + HCl (aq) n 0. 0125 mol 0. 0250 mol c 0. 50 mol/L 1. 0 mol/L V i u xs 0. 0250 L 0. 0125 mol÷ 1 x 1 0. 0250 mol 0. 0125 mol 0 0. 0125 mol [HCl] = n / V = 0. 0125 mol / 0. 050 L = 0. 25 mol/L Na. Cl(aq) n=cx. V + H 2 O (l) HCl (aq) H+ + Clp. H = - log [H+] = - log [0. 25 mol/L] = 0. 602059991 = 0. 60 the [HCl] is 0. 25 mol/L, and the p. H of the final solution is 0. 60.
CAN I … … use stoichiometry to calculate volumes and concentrations in a neutralization reaction? HOMEWORK p 466 #1, 2, 4, 5 p 470 #1 -9
- Slides: 13